A Phase 2A Trial of the Safety and Tolerability of Increased Dose Rifampicin and Adjunctive Linezolid, With or Without Aspirin, for Human Immunodeficiency Virus-Associated Tuberculous Meningitis: The LASER-TBM Trial
- PMID: 36482216
- PMCID: PMC10110270
- DOI: 10.1093/cid/ciac932
A Phase 2A Trial of the Safety and Tolerability of Increased Dose Rifampicin and Adjunctive Linezolid, With or Without Aspirin, for Human Immunodeficiency Virus-Associated Tuberculous Meningitis: The LASER-TBM Trial
Abstract
Background: Drug regimens that include intensified antibiotics alongside effective anti-inflammatory therapies may improve outcomes in tuberculous meningitis (TBM). Safety data on their use in combination and in the context of human immunodeficiency virus (HIV) are needed to inform clinical trial design.
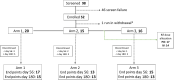
Methods: We conducted a phase 2, open-label, parallel-design, randomized, controlled trial to assess the safety of high-dose rifampicin, linezolid, and high-dose aspirin in HIV-associated TBM. Participants were randomized (1.4:1:1) to 3 treatment arms (1, standard of care [SOC]; 2, SOC + additional rifampicin [up to 35 mg/kg/d] + linezolid 1200 mg/d reducing after 28 days to 600 mg/d; 3, as per arm 2 + aspirin 1000 mg/d) for 56 days, when the primary outcome of adverse events of special interest (AESI) or death was assessed.
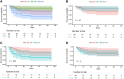
Results: A total of 52 participants with HIV-associated TBM were randomized; 59% had mild disease (British Medical Research Council (MRC) grade 1) vs 39% (grade 2) vs 2% (grade 3). AESI or death occurred in 10 of 16 (63%; arm 3) vs 4 of 14 (29%; arm 2) vs 6 of 20 (30%; arm 1; P = .083). The cumulative proportion of AESI or death (Kaplan-Meier) demonstrated worse outcomes in arm 3 vs arm 1 (P = .04); however, only 1 event in arm 3 was attributable to aspirin and was mild. There was no difference in efficacy (modified Rankin scale) between arms.
Conclusions: High-dose rifampicin and adjunctive linezolid can safely be added to the standard of care in HIV-associated TBM. Larger studies are required to determine whether potential toxicity associated with these interventions, particularly high-dose aspirin, is outweighed by mortality or morbidity benefit.
Clinical trials registration: NCT03927313.
Keywords: HIV; aspirin; linezolid; rifampicin; tuberculous meningitis.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. S. W. reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Pfizer for management of gram-negative infections and participation on the AIDS Clinical Trials Group. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures




References
-
- Lammie GA, Hewlett RH, Schoeman JF, Donald PR. Tuberculous cerebrovascular disease: a review. J Infect 2009; 59:156–66. - PubMed
-
- Misra UK, Kumar M, Kalita J. Seizures in tuberculous meningitis. Epilepsy Res 2018; 148:90–5. - PubMed
-
- Marais S, Roos I, Mitha A, Mabusha SJ, Patel V, Bhigjee AI. Spinal tuberculosis: clinicoradiological findings in 274 patients. Clin Infect Dis 2018; 67:89–98. - PubMed

