Pitfalls in breast pathology
- PMID: 36482276
- PMCID: PMC10107929
- DOI: 10.1111/his.14799
Pitfalls in breast pathology
Abstract
Accurate pathological diagnosis is the cornerstone of optimal clinical management for patients with breast disease. As non-operative diagnosis has now become the standard of care, histopathologists encounter the daily challenge of making definitive diagnoses on limited breast core needle biopsy (CNB) material. CNB samples are carefully evaluated using microscopic examination of haematoxylin and eosin (H&E)-stained slides and supportive immunohistochemistry (IHC), providing the necessary information to inform the next steps in the patient care pathway. Some entities may be difficult to distinguish on small tissue samples, and if there is uncertainty a diagnostic excision biopsy should be recommended. This review discusses (1) benign breast lesions that may mimic malignancy, (2) malignant conditions that may be misinterpreted as benign, (3) malignant conditions that may be incorrectly diagnosed as primary breast carcinoma, and (4) some IHC pitfalls. The aim of the review is to raise awareness of potential pitfalls in the interpretation of breast lesions that may lead to underdiagnosis, overdiagnosis, or incorrect classification of malignancy with potential adverse outcomes for individual patients.
Keywords: breast cancer mimics; immunohistochemistry pitfalls; overdiagnosis; underdiagnosis.
© 2022 The Authors. Histopathology published by John Wiley & Sons Ltd.
Conflict of interest statement
None.
Figures
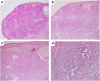
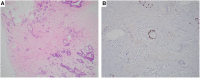

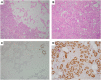

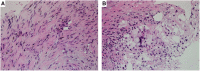
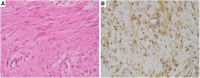

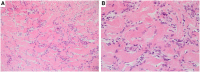


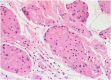
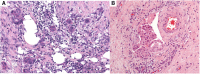
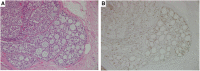

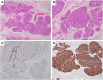






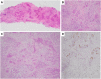
References
-
- Azzopardi JG. Problems in breast pathology. London: Saunders, 1979. - PubMed
-
- Li Z, Dabbs DJ. Avoiding "false positive" and "false negative" immunohistochemical results in breast pathology. Pathobiology 2022; 1‐15; 1–15. - PubMed
-
- Shaaban AM. Diagnostic pitfalls in needle core biopsy of the breast. Diagn. Histopathol. 2022; 28; 156–160.
-
- Kim WG, Cummings MC, Lakhani SR. Pitfalls and controversies in pathology impacting breast cancer management. Expert Rev. Anticancer Ther. 2020; 20; 205–219. - PubMed
-
- Harrison BT, Dillon DA, Richardson AL, Brock JE, Guidi AJ, Lester SC. Quality assurance in breast pathology: lessons learned from a review of amended reports. Arch. Pathol. Lab. Med. 2017; 141; 260–266. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

