Targeting cell death pathways for cancer therapy: recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research
- PMID: 36482419
- PMCID: PMC9733270
- DOI: 10.1186/s13045-022-01392-3
Targeting cell death pathways for cancer therapy: recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research
Abstract
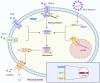
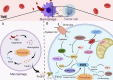
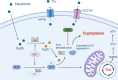
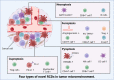
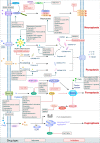
Many types of human cells self-destruct to maintain biological homeostasis and defend the body against pathogenic substances. This process, called regulated cell death (RCD), is important for various biological activities, including the clearance of aberrant cells. Thus, RCD pathways represented by apoptosis have increased in importance as a target for the development of cancer medications in recent years. However, because tumor cells show avoidance to apoptosis, which causes treatment resistance and recurrence, numerous studies have been devoted to alternative cancer cell mortality processes, namely necroptosis, pyroptosis, ferroptosis, and cuproptosis; these RCD modalities have been extensively studied and shown to be crucial to cancer therapy effectiveness. Furthermore, evidence suggests that tumor cells undergoing regulated death may alter the immunogenicity of the tumor microenvironment (TME) to some extent, rendering it more suitable for inhibiting cancer progression and metastasis. In addition, other types of cells and components in the TME undergo the abovementioned forms of death and induce immune attacks on tumor cells, resulting in enhanced antitumor responses. Hence, this review discusses the molecular processes and features of necroptosis, pyroptosis, ferroptosis, and cuproptosis and the effects of these novel RCD modalities on tumor cell proliferation and cancer metastasis. Importantly, it introduces the complex effects of novel forms of tumor cell death on the TME and the regulated death of other cells in the TME that affect tumor biology. It also summarizes the potential agents and nanoparticles that induce or inhibit novel RCD pathways and their therapeutic effects on cancer based on evidence from in vivo and in vitro studies and reports clinical trials in which RCD inducers have been evaluated as treatments for cancer patients. Lastly, we also summarized the impact of modulating the RCD processes on cancer drug resistance and the advantages of adding RCD modulators to cancer treatment over conventional treatments.
Keywords: Cuproptosis; Ferroptosis; Nanoparticles; Necroptosis; Pyroptosis; Tumor microenvironment.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Koren E, Fuchs Y. Modes of regulated cell death in cancer. Cancer Discov. 2021;11(2):245–265. doi: 10.1158/2159-8290.CD-20-0789. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

