A novel viral vaccine platform based on engineered transfer RNA
- PMID: 36482724
- PMCID: PMC9769134
- DOI: 10.1080/22221751.2022.2157339
A novel viral vaccine platform based on engineered transfer RNA
Abstract
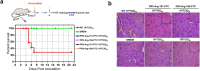
In recent years, an increasing number of emerging and remerging virus outbreaks have occurred and the rapid development of vaccines against these viruses has been crucial. Controlling the replication of premature termination codon (PTC)-containing viruses is a promising approach to generate live but replication-defective viruses that can be used for potent vaccines. Here, we used anticodon-engineered transfer RNAs (ACE-tRNAs) as powerful precision switches to control the replication of PTC-containing viruses. We showed that ACE-tRNAs display higher potency of reading through PTCs than genetic code expansion (GCE) technology. Interestingly, ACE-tRNA has a site preference that may influence its read-through efficacy. We further attempted to use ACE-tRNAs as a novel viral vaccine platform. Using a human immunodeficiency virus type 1 (HIV-1) pseudotyped virus as an RNA virus model, we found that ACE-tRNAs display high potency for read-through viral PTCs and precisely control their production. Pseudorabies virus (PRV), a herpesvirus, was used as a DNA virus model. We found that ACE-tRNAs display high potency for reading through viral PTCs and precisely controlling PTC-containing virus replication. In addition, PTC-engineered PRV completely attenuated and lost virulence in mice in vivo, and immunization with PRV containing a PTC elicited a robust immune response and provided complete protection against wild-type PRV challenge. Overall, replication-controllable PTC-containing viruses based on ACE-tRNAs provide a new strategy to rapidly attenuate virus infection and prime robust immune responses. This technology can be used as a platform for rapidly developing viral vaccines in the future.
Keywords: Engineered transfer RNA; PTC virus; Pseudorabies virus; novel vaccine; switch; vaccine.
Conflict of interest statement
Yan-Dong Tang and Xue-Hui Cai filed two patents related to this technology.
Figures


Similar articles
-
Generation of Premature Termination Codon (PTC)-Harboring Pseudorabies Virus (PRV) via Genetic Code Expansion Technology.Viruses. 2022 Mar 10;14(3):572. doi: 10.3390/v14030572. Viruses. 2022. PMID: 35336979 Free PMC article.
-
Characterization of a quadruple glycoprotein-deleted pseudorabies virus mutant for use as a biologically safe live virus vaccine.J Gen Virol. 1994 Jul;75 ( Pt 7):1723-33. doi: 10.1099/0022-1317-75-7-1723. J Gen Virol. 1994. PMID: 8021601
-
An approach to a FMD vaccine based on genetic engineered attenuated pseudorabies virus: one experiment using VP1 gene alone generates an antibody responds on FMD and pseudorabies in swine.Vaccine. 2004 Jun 2;22(17-18):2129-36. doi: 10.1016/j.vaccine.2003.12.005. Vaccine. 2004. PMID: 15149769 Clinical Trial.
-
Vaccines against pseudorabies virus (PrV).Vet Microbiol. 2017 Jul;206:3-9. doi: 10.1016/j.vetmic.2016.11.019. Epub 2016 Nov 18. Vet Microbiol. 2017. PMID: 27890448 Review.
-
Pseudorabies virus infections in pigs. Role of viral proteins in virulence, pathogenesis and transmission.Vet Res. 1997;28(1):1-17. Vet Res. 1997. PMID: 9172836 Review.
Cited by
-
Advancing Therapeutic Strategies for Nonsense-Related Diseases: From Small Molecules to Nucleic Acid-Based Innovations.IUBMB Life. 2025 May;77(5):e70027. doi: 10.1002/iub.70027. IUBMB Life. 2025. PMID: 40420818 Free PMC article. Review.
-
Generation of an infectious cDNA clone for NADC30-like PRRSV.Front Vet Sci. 2024 Aug 14;11:1468981. doi: 10.3389/fvets.2024.1468981. eCollection 2024. Front Vet Sci. 2024. PMID: 39205805 Free PMC article.
-
PROTAC-Based Antivirals for Respiratory Viruses: A Novel Approach for Targeted Therapy and Vaccine Development.Microorganisms. 2025 Jul 2;13(7):1557. doi: 10.3390/microorganisms13071557. Microorganisms. 2025. PMID: 40732065 Free PMC article. Review.
-
Minor envelope proteins from GP2a to GP4 contribute to the spread pattern and yield of type 2 PRRSV in MARC-145 cells.Front Cell Infect Microbiol. 2024 Mar 25;14:1376725. doi: 10.3389/fcimb.2024.1376725. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38590440 Free PMC article.
-
Viral Live-Attenuated Vaccines (LAVs): Past and Future Directions.Adv Sci (Weinh). 2025 Jan;12(3):e2407241. doi: 10.1002/advs.202407241. Epub 2024 Dec 6. Adv Sci (Weinh). 2025. PMID: 39639853 Free PMC article. Review.
References
-
- Drosten C, Gunther S, Preiser W, et al. . Identification of a novel coronavirus in patients with severe acute respiratory syndrome [research support, non-U.S. gov't]. N Engl J Med. 2003 May 15;348(20):1967–1976. - PubMed
-
- Peiris JS, Yuen KY, Osterhaus AD, et al. . The severe acute respiratory syndrome [review]. N Engl J Med. 2003 Dec 18;349(25):2431–2441. - PubMed
-
- Zaki AM, van Boheemen S, Bestebroer TM, et al. . Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia [case reports research support, non-U.S. gov't]. N Engl J Med. 2012 Nov 8;367(19):1814–1820. - PubMed
-
- Baize S, Pannetier D, Oestereich L, et al. . Emergence of Zaire ebola virus disease in Guinea [research support, non-U.S. gov't]. N Engl J Med. 2014 Oct 9;371(15):1418–1425. - PubMed
-
- Petersen LR, Jamieson DJ, Powers AM, et al. . Zika virus [historical article review]. N Engl J Med. 2016 Apr 21;374(16):1552–1563. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
