RNA in Therapeutics: CRISPR in the Clinic
- PMID: 36482771
- PMCID: PMC9880604
- DOI: 10.14348/molcells.2022.0163
RNA in Therapeutics: CRISPR in the Clinic
Abstract
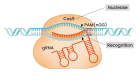
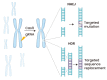
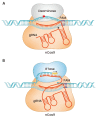
The advent of the CRISPR-Cas genome editing platform has greatly enhanced the capabilities of researchers in many areas of biology. Its use has also been turned to the development of therapies for genetic diseases and to the enhancement of cell therapies. This review describes some recent advances in these areas.
Keywords: CRISPR; Cas9; base editing; genome editing; prime editing; sickle cell disease.
Conflict of interest statement
D.C. receives license royalties from Sangamo Therapeutics for use of zinc finger nucleases in genome editing.
Figures
Similar articles
-
Advances in CRISPR/Cas-based Gene Therapy in Human Genetic Diseases.Theranostics. 2020 Mar 15;10(10):4374-4382. doi: 10.7150/thno.43360. eCollection 2020. Theranostics. 2020. PMID: 32292501 Free PMC article. Review.
-
High content analysis platform for optimization of lipid mediated CRISPR-Cas9 delivery strategies in human cells.Acta Biomater. 2016 Apr 1;34:143-158. doi: 10.1016/j.actbio.2015.12.036. Epub 2015 Dec 30. Acta Biomater. 2016. PMID: 26747759 Free PMC article.
-
CRISPR/Cas9 for Human Genome Engineering and Disease Research.Annu Rev Genomics Hum Genet. 2016 Aug 31;17:131-54. doi: 10.1146/annurev-genom-083115-022258. Epub 2016 May 23. Annu Rev Genomics Hum Genet. 2016. PMID: 27216776 Free PMC article. Review.
-
CRISPR-Cas System: The Current and Emerging Translational Landscape.Cells. 2023 Apr 7;12(8):1103. doi: 10.3390/cells12081103. Cells. 2023. PMID: 37190012 Free PMC article. Review.
-
CRISPR-cas9 genome editing delivery systems for targeted cancer therapy.Life Sci. 2021 Feb 15;267:118969. doi: 10.1016/j.lfs.2020.118969. Epub 2020 Dec 29. Life Sci. 2021. PMID: 33385410 Review.
Cited by
-
CircRNA-based AntimiR therapy: A novel approach to hypertension treatment.Noncoding RNA Res. 2025 May 5;13:94-108. doi: 10.1016/j.ncrna.2025.05.001. eCollection 2025 Aug. Noncoding RNA Res. 2025. PMID: 40487299 Free PMC article. Review.
-
A brief guide for gene delivery to the brain using adeno-associated viral vectors.Mol Cells. 2025 Mar;48(3):100189. doi: 10.1016/j.mocell.2025.100189. Epub 2025 Feb 2. Mol Cells. 2025. PMID: 39904462 Free PMC article. Review.
-
Delivery Strategies of siRNA Therapeutics for Hair Loss Therapy.Int J Mol Sci. 2024 Jul 11;25(14):7612. doi: 10.3390/ijms25147612. Int J Mol Sci. 2024. PMID: 39062852 Free PMC article. Review.
-
Dysregulated RNA-binding proteins and alternative splicing: Emerging roles in autism spectrum disorder.Mol Cells. 2025 Aug;48(8):100237. doi: 10.1016/j.mocell.2025.100237. Epub 2025 Jun 3. Mol Cells. 2025. PMID: 40472970 Free PMC article. Review.
-
Guide for generating single-cell-derived knockout clones in mammalian cell lines using the CRISPR/Cas9 system.Mol Cells. 2024 Jul;47(7):100087. doi: 10.1016/j.mocell.2024.100087. Epub 2024 Jun 25. Mol Cells. 2024. PMID: 38936509 Free PMC article.
References
-
- Amoasii L., Hildyard J.C.W., Li H., Sanchez-Ortiz E., Mireault A., Caballero D., Harron R., Stathopoulou T.R., Massey C., Shelton J.M., et al. Gene editing restores dystrophin expression in a canine model of Duchenne muscular dystrophy. Science. 2018;362:86–91. doi: 10.1126/science.aau1549. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

