Selective incorporation of 5-hydroxytryptophan blocks long range electron transfer in oxalate decarboxylase
- PMID: 36482787
- PMCID: PMC9801070
- DOI: 10.1002/pro.4537
Selective incorporation of 5-hydroxytryptophan blocks long range electron transfer in oxalate decarboxylase
Abstract
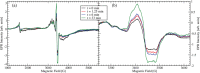
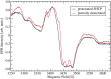
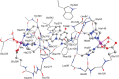
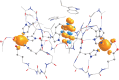
Oxalate decarboxylase from Bacillus subtilis is a binuclear Mn-dependent acid stress response enzyme that converts the mono-anion of oxalic acid into formate and carbon dioxide in a redox neutral unimolecular disproportionation reaction. A π-stacked tryptophan dimer, W96 and W274, at the interface between two monomer subunits facilitates long-range electron transfer between the two Mn ions and plays an important role in the catalytic mechanism. Substitution of W96 with the unnatural amino acid 5-hydroxytryptophan leads to a persistent EPR signal which can be traced back to the neutral radical of 5-hydroxytryptophan with its hydroxyl proton removed. 5-Hydroxytryptophan acts as a hole sink preventing the formation of Mn(III) at the N-terminal active site and strongly suppresses enzymatic activity. The lower boundary of the standard reduction potential for the active site Mn(II)/Mn(III) couple can therefore be estimated as 740 mV against the normal hydrogen electrode at pH 4, the pH of maximum catalytic efficiency. Our results support the catalytic importance of long-range electron transfer in oxalate decarboxylase while at the same time highlighting the utility of unnatural amino acid incorporation and specifically the use of 5-hydroxytryptophan as an energetic sink for hole hopping to probe electron transfer in redox proteins.
Keywords: 5-hydroxytryptophan; density functional theory; electron paramagnetic resonance; genetic code expansion; long range electron transfer; oxalate decarboxylase.
© 2022 The Protein Society.
Conflict of interest statement
There are no conflicts to declare.
Figures
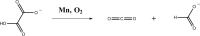
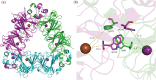
Similar articles
-
Bidentate Substrate Binding Mode in Oxalate Decarboxylase.Molecules. 2024 Sep 17;29(18):4414. doi: 10.3390/molecules29184414. Molecules. 2024. PMID: 39339409 Free PMC article.
-
Oxalate decarboxylase uses electron hole hopping for catalysis.J Biol Chem. 2021 Jul;297(1):100857. doi: 10.1016/j.jbc.2021.100857. Epub 2021 Jun 5. J Biol Chem. 2021. PMID: 34097877 Free PMC article.
-
Redox Cycling, pH Dependence, and Ligand Effects of Mn(III) in Oxalate Decarboxylase from Bacillus subtilis.Biochemistry. 2016 Nov 29;55(47):6505-6516. doi: 10.1021/acs.biochem.6b00891. Epub 2016 Nov 16. Biochemistry. 2016. PMID: 27797181
-
Amino acid residues involved in the coordination and assembly of the manganese cluster of photosystem II. Proton-coupled electron transport of the redox-active tyrosines and its relationship to water oxidation.Biochim Biophys Acta. 2001 Jan 5;1503(1-2):147-63. doi: 10.1016/s0005-2728(00)00220-6. Biochim Biophys Acta. 2001. PMID: 11115631 Review.
-
An evaluation of structural models for the photosynthetic water-oxidizing complex derived from spectroscopic and X-ray diffraction signatures.J Biol Inorg Chem. 2002 Jan;7(1-2):2-22. doi: 10.1007/s00775-001-0305-3. Epub 2001 Nov 8. J Biol Inorg Chem. 2002. PMID: 11862536 Review.
Cited by
-
Cellular Site-Specific Incorporation of Noncanonical Amino Acids in Synthetic Biology.Chem Rev. 2024 Sep 25;124(18):10577-10617. doi: 10.1021/acs.chemrev.3c00938. Epub 2024 Aug 29. Chem Rev. 2024. PMID: 39207844 Review.
-
Bidentate Substrate Binding Mode in Oxalate Decarboxylase.Molecules. 2024 Sep 17;29(18):4414. doi: 10.3390/molecules29184414. Molecules. 2024. PMID: 39339409 Free PMC article.
References
-
- Bae JH, Rubini M, Jung G, Wiegand G, Seifert MHJ, Azim MK, et al. Expansion of the genetic code enables design of a novel “gold” class of green fluorescent proteins. J Mol Biol. 2003;328:1071–81. - PubMed
-
- Barone V, Cossi M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J Phys Chem A. 1998;102:1995–2001.
-
- Becke AD. Density‐functional exchange‐energy approximation with correct asymptotic behavior. Phys Rev A At Mol Opt Phys. 1988;38:3098–100. - PubMed
-
- Becke AD. Density‐functional thermochemistry. III. The role of exact exchange. J Chem Phys. 1993;98:5648–52.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

