The Impact of Reducing Nicotine Content on Adolescent Cigarette Smoking and Nicotine Exposure: Results From a Randomized Controlled Trial
- PMID: 36482794
- PMCID: PMC10077938
- DOI: 10.1093/ntr/ntac279
The Impact of Reducing Nicotine Content on Adolescent Cigarette Smoking and Nicotine Exposure: Results From a Randomized Controlled Trial
Abstract
Introduction: As the science base around the potential benefits of a reduced-nicotine standard for cigarettes grows, information on the potential effects on adolescent smokers is a high priority. The aim of this randomized trial was to test the influence of 3-week exposure to reduced nicotine cigarettes in a sample of adolescent daily smokers.
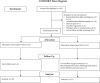
Aims and methods: In this double-blind, two-arm, randomized controlled trial (NCT0258731), following a 1-week baseline, adolescent daily smokers not currently intending to quit (ages 15-19 years, n = 66 randomized) were urn randomized to use either very low nicotine content (VLNC; 0.4 mg/g; n = 33) or normal nicotine content (NNC, 15.8 mg/g; n = 33) research cigarettes for 3 weeks. Participants attended five study sessions at our clinical laboratory. The primary outcome was average total cigarettes smoked per day (CPD; including both study and non-study cigarettes) at week 3.
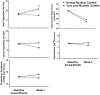
Results: Stepwise regression results demonstrated that compared with NNC cigarettes (n = 31), assignment to VLNC cigarettes (n = 29), was associated with 2.4 fewer CPD on average than NNC assignment (p < .05) week 3 when controlling for covariates (p < .01, Cohen's d = 0.52 n = 60 completed all procedures). VLNC cigarettes were also associated with lower levels of craving reduction than NNC cigarettes (Questionnaire on Smoking Urges Factor 2, p < .05). No group differences were found for secondary outcomes.
Conclusions: Adolescent participants assigned to VLNC use for 3 weeks smoked fewer total CPD relative to the NNC group. Overall, data suggest that a VLNC policy would reduce cigarette smoking in adolescents who smoke, but high rates of incomplete adherence suggest that youth may seek alternative sources of nicotine in this scenario.
Implications: The US Food and Drug Administration may enact a reduced-nicotine product standard that would affect all commercially available cigarettes. One important population affected by this policy would be adolescents who smoke. This study, the first clinical trial of VLNC cigarettes in adolescents, demonstrates that adolescents switched to VLNC cigarettes for 3 weeks reduced their CPD relative to the normal-nicotine cigarette control group, without leading to increased respiratory symptoms or increased withdrawal. Biomarkers indicated the use of other sources of nicotine, suggesting that such a policy will need to consider approaches to assist in transitioning away from smoking.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Society for Research on Nicotine and Tobacco. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
The authors have no conflicts to declare.
Figures
Similar articles
-
Smoking Topography Characteristics During a 6-Week Trial of Very Low Nicotine Content Cigarettes in Smokers With Serious Mental Illness.Nicotine Tob Res. 2020 Jul 16;22(8):1414-1418. doi: 10.1093/ntr/ntz198. Nicotine Tob Res. 2020. PMID: 31628475 Free PMC article. Clinical Trial.
-
Reduced nicotine content cigarettes and use of alternative nicotine products: exploratory trial.Addiction. 2017 Jan;112(1):156-167. doi: 10.1111/add.13603. Epub 2016 Nov 2. Addiction. 2017. PMID: 27614097 Free PMC article. Clinical Trial.
-
Evaluation of a reduced nicotine product standard: Moderating effects of and impact on cannabis use.Drug Alcohol Depend. 2016 Oct 1;167:228-32. doi: 10.1016/j.drugalcdep.2016.08.620. Epub 2016 Aug 28. Drug Alcohol Depend. 2016. PMID: 27590743 Free PMC article. Clinical Trial.
-
The Importance of Estimating Causal Effects for Evaluating a Nicotine Standard for Cigarettes.Nicotine Tob Res. 2019 Dec 23;21(Suppl 1):S22-S25. doi: 10.1093/ntr/ntz119. Nicotine Tob Res. 2019. PMID: 31867648 Free PMC article. Review.
-
A review of the efficacy of smoking-cessation pharmacotherapies in nonwhite populations.Clin Ther. 2008 May;30(5):800-12. doi: 10.1016/j.clinthera.2008.05.010. Clin Ther. 2008. PMID: 18555928 Review.
Cited by
-
Reduced nicotine in cigarettes in a marketplace with alternative nicotine systems: randomized clinical trial.Lancet Reg Health Am. 2024 Jun 3;35:100796. doi: 10.1016/j.lana.2024.100796. eCollection 2024 Jul. Lancet Reg Health Am. 2024. PMID: 38911348 Free PMC article.
-
Public Perceptions of Very Low Nicotine Content on Twitter: Observational Study.JMIR Form Res. 2024 Dec 4;8:e63035. doi: 10.2196/63035. JMIR Form Res. 2024. PMID: 39631065 Free PMC article.
-
Reactions to a Nicotine Reduction Policy Among Adolescents Who Smoke: A Qualitative Study.Nicotine Tob Res. 2024 Nov 22;26(12):1692-1699. doi: 10.1093/ntr/ntae153. Nicotine Tob Res. 2024. PMID: 38908011 Free PMC article. Clinical Trial.
-
Racial/ethnic differences in the acute effects of reduced nicotine content cigarettes among adolescents who smoke.Addict Behav. 2025 Jan;160:108147. doi: 10.1016/j.addbeh.2024.108147. Epub 2024 Sep 7. Addict Behav. 2025. PMID: 39243729
-
Qualitative reactions to a low nicotine product standard for cigarettes from adolescents and young adults living in the United States who smoke.Prev Med Rep. 2023 Feb 23;32:102163. doi: 10.1016/j.pmedr.2023.102163. eCollection 2023 Apr. Prev Med Rep. 2023. PMID: 36895826 Free PMC article.
References
-
- Tobacco Product Standard for Nicotine Level of Combusted Cigarettes. Federal Register ; 2018. https://www.federalregister.gov/documents/2018/03/16/2018-05345/tobacco-.... Accessed May 5, 2022.
-
- Food And Drug Administration. FDA Announces Plans for Proposed Rule to Reduce Addictiveness of Cigarettes and Other Combusted Tobacco Products. FDA; 2022. https://www.fda.gov/news-events/press-announcements/fda-announces-plans-.... Accessed October 19, 2022.
-
- Apelberg BJ, Feirman SP, Salazar E, et al. Potential public health effects of reducing nicotine levels in cigarettes in the United States. N Engl J Med. 2018;378(18):1725–1733. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials