Fabrication of gentamicin loaded Col-I/HA multilayers modified titanium coatings for prevention of implant infection
- PMID: 36482941
- PMCID: PMC9722959
- DOI: 10.3389/fchem.2022.1019332
Fabrication of gentamicin loaded Col-I/HA multilayers modified titanium coatings for prevention of implant infection
Abstract
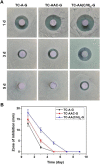
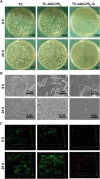
In this study, gentamicin loaded collagen I/hyaluronic acid multilayers modified titanium coating (TC-AA(C/H)6-G) was fabricated via a layer-by-layer (LBL) covalent immobilization method. The drug releasing properties of collagen I/Hyaluronic acid (Col-I/HA) multilayers and the effect of loaded gentamicin on the antibacterial properties and cytocompatibility of modified TC were investigated. The gentamicin release assay indicated that the Col-I/HA multilayers modified TC exhibited agreeable drug-loading amount (537.22 ± 29.66 µg of gentamicin) and controlled-release performance (240 h of sustained release time). TC-AA(C/H)6-G revealed satisfactory antibacterial activity and inhibited the colonization and biofilm formation of S. aureus. Fortunately, the functions of hMSCs on TC-AA(C/H)6-G did not affected by the loaded gentamicin, and TC-AA(C/H)6-G could improve the adhesion, proliferation and osteogenic differentiation of cells, as well as TC-AA(C/H)6. In vivo animal study indicated that TC-AA(C/H)6-G could effectively control intramedullary cavity infection caused by S. aureus and prevent bone destruction.
Keywords: Col-I/HA multilayers; cytocompatibility; drug-release; prevention infection; titanium coatings.
Copyright © 2022 Ma, Zong, Xun, Hu, Chen, Zhang, Peng, Song and Ao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








Similar articles
-
Evaluation of collagen/hydroxyapatite electrospun layers loaded with vancomycin, gentamicin and their combination: Comparison of release kinetics, antimicrobial activity and cytocompatibility.Eur J Pharm Biopharm. 2019 Jul;140:50-59. doi: 10.1016/j.ejpb.2019.04.021. Epub 2019 May 2. Eur J Pharm Biopharm. 2019. PMID: 31055065
-
An in vivo study on the effect of coating stability on osteointegration performance of collagen/hyaluronic acid multilayer modified titanium implants.Bioact Mater. 2017 Jul 27;3(1):97-101. doi: 10.1016/j.bioactmat.2017.07.004. eCollection 2018 Mar. Bioact Mater. 2017. PMID: 29744446 Free PMC article.
-
Fabrication and in vitro evaluation of stable collagen/hyaluronic acid biomimetic multilayer on titanium coatings.J R Soc Interface. 2013 May 1;10(84):20130070. doi: 10.1098/rsif.2013.0070. Print 2013 Jul 6. J R Soc Interface. 2013. PMID: 23635490 Free PMC article.
-
Layer-by-layer self-assembly of minocycline-loaded chitosan/alginate multilayer on titanium substrates to inhibit biofilm formation.J Dent. 2014 Nov;42(11):1464-72. doi: 10.1016/j.jdent.2014.06.003. Epub 2014 Jun 12. J Dent. 2014. PMID: 24930872
-
Antibacterial Layer-by-Layer Coatings for Medical Implants.Pharmaceutics. 2020 Dec 24;13(1):16. doi: 10.3390/pharmaceutics13010016. Pharmaceutics. 2020. PMID: 33374184 Free PMC article. Review.
Cited by
-
Cationized Decalcified Bone Matrix for Infected Bone Defect Treatment.BME Front. 2024 Oct 2;5:0066. doi: 10.34133/bmef.0066. eCollection 2024. BME Front. 2024. PMID: 39360181 Free PMC article.
-
The Impact of Implant Surface Modifications on the Osseointegration Process: An Overview.Cureus. 2025 Apr 1;17(4):e81576. doi: 10.7759/cureus.81576. eCollection 2025 Apr. Cureus. 2025. PMID: 40177230 Free PMC article. Review.
References
-
- Ao H., Yang S., Nie B. e., Fan Q., Zhang Q., Zong J., et al. (2019). Improved antibacterial properties of collagen I/hyaluronic acid/quaternized chitosan multilayer modified titanium coatings with both contact-killing and release-killing functions. J. Mater. Chem. B 7, 1951–1961. 10.1039/c8tb02425a - DOI - PubMed
LinkOut - more resources
Full Text Sources

