The role of Nrf2 in periodontal disease by regulating lipid peroxidation, inflammation and apoptosis
- PMID: 36482997
- PMCID: PMC9723463
- DOI: 10.3389/fendo.2022.963451
The role of Nrf2 in periodontal disease by regulating lipid peroxidation, inflammation and apoptosis
Abstract
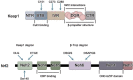
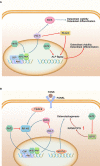
Nuclear factor E2-related factor 2(Nrf2) is a transcription factor that mainly regulates oxidative stress in the body. It initiates the expression of several downstream antioxidants, anti-inflammatory proteins and detoxification enzymes through the Kelch-like ECH-associating protein 1 (Keap1) -nuclear factor E2-related factor 2(Nrf2) -antioxidant response element (ARE) signaling pathway. Its anti-apoptosis, anti-oxidative stress and anti-inflammatory effects have gradually become the focus of periodontal disease research in recent years. In this paper, the structure and function of Nrf2 pathway and its mechanism of action in the treatment of periodontitis in recent years were analyzed and summarized, so as to further clarify the relationship between Nrf2 pathway and oxidative stress in the occurrence and development of periodontitis, and to provide ideas for the development of new treatment drugs targeting Nrf2 pathway.
Keywords: Nrf2; apoptosis; bone homeostasis; lipid peroxidation; periodontal disease.
Copyright © 2022 Ma, Luo, Lu, Jiang, Chen, Deng, Ma and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Comment in
-
Editorial: The role of oxidative stress in metabolic and inflammatory diseases.Front Endocrinol (Lausanne). 2024 Feb 8;15:1374584. doi: 10.3389/fendo.2024.1374584. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38390210 Free PMC article. No abstract available.
Similar articles
-
The anti-inflammatory and anti-oxidative effect of a classical hypnotic bromovalerylurea mediated by the activation of NRF2.J Biochem. 2023 Jul 31;174(2):131-142. doi: 10.1093/jb/mvad030. J Biochem. 2023. PMID: 37039781 Free PMC article.
-
Lycium barbarum polysaccharides attenuate cardiovascular oxidative stress injury by enhancing the Keap1/Nrf2 signaling pathway in exhaustive exercise rats.Mol Med Rep. 2021 Sep;24(3):643. doi: 10.3892/mmr.2021.12282. Epub 2021 Jul 19. Mol Med Rep. 2021. PMID: 34278476
-
Activation of kelch-like ECH-associated protein 1/nuclear factor erythroid 2-related factor 2/antioxidant response element pathway by curcumin enhances the anti-oxidative capacity of corneal endothelial cells.Biomed Pharmacother. 2021 Sep;141:111834. doi: 10.1016/j.biopha.2021.111834. Epub 2021 Jun 18. Biomed Pharmacother. 2021. PMID: 34153850
-
Stress-sensing mechanisms and the physiological roles of the Keap1-Nrf2 system during cellular stress.J Biol Chem. 2017 Oct 13;292(41):16817-16824. doi: 10.1074/jbc.R117.800169. Epub 2017 Aug 24. J Biol Chem. 2017. PMID: 28842501 Free PMC article. Review.
-
The Keap1-Nrf2-ARE Pathway As a Potential Preventive and Therapeutic Target: An Update.Med Res Rev. 2016 Sep;36(5):924-63. doi: 10.1002/med.21396. Epub 2016 May 18. Med Res Rev. 2016. PMID: 27192495 Review.
Cited by
-
The Impact of Ozone on Periodontal Cell Line Viability and Function.Curr Issues Mol Biol. 2025 Jan 23;47(2):72. doi: 10.3390/cimb47020072. Curr Issues Mol Biol. 2025. PMID: 39996793 Free PMC article. Review.
-
New Insights in Natural Bioactive Compounds for Periodontal Disease: Advanced Molecular Mechanisms and Therapeutic Potential.Molecules. 2025 Feb 10;30(4):807. doi: 10.3390/molecules30040807. Molecules. 2025. PMID: 40005119 Free PMC article. Review.
-
The Role of Nrf2 in the Regulation of Periodontitis, Peri-implantitis, Dentin Infection, and Apical Periodontitis.Biol Proced Online. 2025 Jul 2;27(1):23. doi: 10.1186/s12575-025-00285-2. Biol Proced Online. 2025. PMID: 40604430 Free PMC article. Review.
-
Role of Oxidative Stress Signaling, Nrf2, on Survival and Stemness of Human Adipose-Derived Stem Cells Exposed to X-rays, Protons and Carbon Ions.Antioxidants (Basel). 2024 Aug 26;13(9):1035. doi: 10.3390/antiox13091035. Antioxidants (Basel). 2024. PMID: 39334694 Free PMC article.
-
Ferroptosis and cuproptosis in periodontitis: recent biological insights and therapeutic advances.Front Immunol. 2025 Feb 24;16:1526961. doi: 10.3389/fimmu.2025.1526961. eCollection 2025. Front Immunol. 2025. PMID: 40066457 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

