Epigenetic therapy reprograms M2-type tumor-associated macrophages into an M1-like phenotype by upregulating miR-7083-5p
- PMID: 36483544
- PMCID: PMC9724234
- DOI: 10.3389/fimmu.2022.976196
Epigenetic therapy reprograms M2-type tumor-associated macrophages into an M1-like phenotype by upregulating miR-7083-5p
Abstract
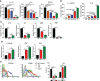
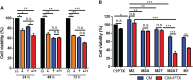
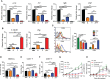
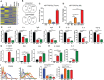
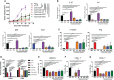
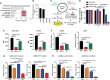
Reprogramming M2-type, pro-tumoral tumor-associated macrophages (TAMs) into M1-type, anti-tumoral macrophages is a key strategy in cancer therapy. In this study, we exploited epigenetic therapy using the DNA methylation inhibitor 5-aza-2'-deoxycytidine (5-aza-dC) and the histone deacetylation inhibitor trichostatin A (TSA), to reprogram M2-type macrophages into an M1-like phenotype. Treatment of M2-type macrophages with the combination of 5-aza-dC and TSA decreased the levels of M2 macrophage cytokines while increasing those of M1 macrophage cytokines, as compared to the use of either therapy alone. Conditioned medium of M2 macrophages treated with the combination of 5-aza-dC and TSA sensitized the tumor cells to paclitaxel. Moreover, treatment with the combination inhibited tumor growth and improved anti-tumor immunity in the tumor microenvironment. Depletion of macrophages reduced the anti-tumor growth activity of the combination therapy. Profiling of miRNAs revealed that the expression of miR-7083-5p was remarkably upregulated in M2 macrophages, following treatment with 5-aza-dC and TSA. Transfection of miR-7083-5p reprogrammed the M2-type macrophages towards an M1-like phenotype, and adoptive transfer of M2 macrophages pre-treated with miR-7083-5p into mice inhibited tumor growth. miR-7083-5p inhibited the expression of colony-stimulating factor 2 receptor alpha and CD43 as candidate targets. These results show that epigenetic therapy upon treatment with the combination of 5-aza-dC and TSA skews M2-type TAMs towards the M1-like phenotype by upregulating miR-7083-5p, which contributes to the inhibition of tumor growth.
Keywords: 5-aza-2’-deoxycytidine; epigenetic therapy; macrophage reprogramming; miR-7083-5p; trichostatin A.
Copyright © 2022 Vadevoo, Gunassekaran, Yoo, Kwon, Hur, Chae and Lee.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

