Pediatric versus adult high grade glioma: Immunotherapeutic and genomic considerations
- PMID: 36483545
- PMCID: PMC9722734
- DOI: 10.3389/fimmu.2022.1038096
Pediatric versus adult high grade glioma: Immunotherapeutic and genomic considerations
Abstract
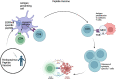
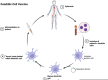
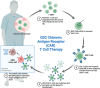
High grade gliomas are identified as malignant central nervous tumors that spread rapidly and have a universally poor prognosis. Historically high grade gliomas in the pediatric population have been treated similarly to adult high grade gliomas. For the first time, the most recent classification of central nervous system tumors by World Health Organization has divided adult from pediatric type diffuse high grade gliomas, underscoring the biologic differences between these tumors in different age groups. The objective of our review is to compare high grade gliomas in the adult versus pediatric patient populations, highlighting similarities and differences in epidemiology, etiology, pathogenesis and therapeutic approaches. High grade gliomas in adults versus children have varying clinical presentations, molecular biology background, and response to chemotherapy, as well as unique molecular targets. However, increasing evidence show that they both respond to recently developed immunotherapies. This review summarizes the distinctions and commonalities between the two in disease pathogenesis and response to therapeutic interventions with a focus on immunotherapy.
Keywords: adult; glioblastoma; high grade glioma; immunotherapy; pediatric.
Copyright © 2022 Aggarwal, Luo, Pehlivan, Hoang, Rajappa, Cripe, Cassady, Lee and Cairo.
Conflict of interest statement
MC serves as a consultant for Jazz, Omeros, Astra Zeneca, Nektar Therapeutics and Novartis and a member of the Speakers Bureau for Jazz, Servier, Amgen, Sanofi and Sobi. DL reports personal fees and other from Kiadis Pharma, CytoSen Therapeutics, Courier Therapeutics, and Caribou Biosciences outside the submitted work; In addition, DL has a patent broadly related to NK cell therapy of cancer with royalties paid to Kiadis Pharma. TC recently served as a one-time consultant to Blueprint, Incyte, Oncopeptides, serves as a DSMB chair for SpringWorks and is a cofounder of Vironexis Biotherapeutics, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

