CAR-T cell therapy in triple-negative breast cancer: Hunting the invisible devil
- PMID: 36483567
- PMCID: PMC9722775
- DOI: 10.3389/fimmu.2022.1018786
CAR-T cell therapy in triple-negative breast cancer: Hunting the invisible devil
Abstract
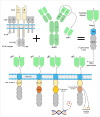
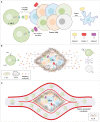
Triple-negative breast cancer (TNBC) is known as the most intricate and hard-to-treat subtype of breast cancer. TNBC cells do not express the well-known estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 (HER2) expressed by other breast cancer subtypes. This phenomenon leaves no room for novel treatment approaches including endocrine and HER2-specific antibody therapies. To date, surgery, radiotherapy, and systemic chemotherapy remain the principal therapy options for TNBC treatment. However, in numerous cases, these approaches either result in minimal clinical benefit or are nonfunctional, resulting in disease recurrence and poor prognosis. Nowadays, chimeric antigen receptor T cell (CAR-T) therapy is becoming more established as an option for the treatment of various types of hematologic malignancies. CAR-Ts are genetically engineered T lymphocytes that employ the body's immune system mechanisms to selectively recognize cancer cells expressing tumor-associated antigens (TAAs) of interest and efficiently eliminate them. However, despite the clinical triumph of CAR-T therapy in hematologic neoplasms, CAR-T therapy of solid tumors, including TNBC, has been much more challenging. In this review, we will discuss the success of CAR-T therapy in hematological neoplasms and its caveats in solid tumors, and then we summarize the potential CAR-T targetable TAAs in TNBC studied in different investigational stages.
Keywords: T-cell therapy; adoptive cell therapy; cancer immunotherapy; chimeric antigen receptor; solid tumors; triple-negative breast cancer.
Copyright © 2022 Nasiri, Kazemi, Mirarefin, Mahboubi Kancha, Ahmadi Najafabadi, Salem, Dashti Shokoohi, Evazi Bakhshi, Safarzadeh Kozani and Safarzadeh Kozani.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Emerging CAR-T Cell Therapy for the Treatment of Triple-Negative Breast Cancer.Mol Cancer Ther. 2020 Dec;19(12):2409-2421. doi: 10.1158/1535-7163.MCT-20-0385. Epub 2020 Oct 21. Mol Cancer Ther. 2020. PMID: 33087511 Review.
-
Expression of CD22 in Triple-Negative Breast Cancer: A Novel Prognostic Biomarker and Potential Target for CAR Therapy.Int J Mol Sci. 2023 Jan 21;24(3):2152. doi: 10.3390/ijms24032152. Int J Mol Sci. 2023. PMID: 36768478 Free PMC article.
-
CAR T-cell therapy for triple-negative breast cancer: Where we are.Cancer Lett. 2020 Oct 28;491:121-131. doi: 10.1016/j.canlet.2020.07.044. Epub 2020 Aug 12. Cancer Lett. 2020. PMID: 32795486 Review.
-
Novel chimeric antigen receptor T cell-based immunotherapy: a perspective for triple-negative breast cancer.Front Cell Dev Biol. 2023 Jun 29;11:1158539. doi: 10.3389/fcell.2023.1158539. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37457288 Free PMC article. Review.
-
CAR T Cells Targeting the Tumor MUC1 Glycoprotein Reduce Triple-Negative Breast Cancer Growth.Front Immunol. 2019 May 24;10:1149. doi: 10.3389/fimmu.2019.01149. eCollection 2019. Front Immunol. 2019. PMID: 31178870 Free PMC article.
Cited by
-
Journey of CAR T‑cells: Emphasising the concepts and advancements in breast cancer (Review).Int J Oncol. 2023 Dec;63(6):130. doi: 10.3892/ijo.2023.5578. Epub 2023 Oct 13. Int J Oncol. 2023. PMID: 37830150 Free PMC article. Review.
-
Pentoxifylline changes the balance of immune cell population in breast tumor-infiltrating lymphocytes.Med Oncol. 2023 May 6;40(6):168. doi: 10.1007/s12032-023-02034-5. Med Oncol. 2023. PMID: 37149505 Free PMC article.
-
Prognostic significance of peripheral and tumor-infiltrating lymphocytes in newly diagnosed stage III/IV non-small-cell lung cancer.Front Med (Lausanne). 2024 May 22;11:1349178. doi: 10.3389/fmed.2024.1349178. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38841570 Free PMC article.
-
Targeting Interleukin-13 Receptor α2 and EphA2 in Aggressive Breast Cancer Subtypes with Special References to Chimeric Antigen Receptor T-Cell Therapy.Int J Mol Sci. 2024 Mar 28;25(7):3780. doi: 10.3390/ijms25073780. Int J Mol Sci. 2024. PMID: 38612592 Free PMC article. Review.
-
Unveiling the Immune Microenvironment's Role in Breast Cancer: A Glimpse into Promising Frontiers.Int J Mol Sci. 2023 Oct 18;24(20):15332. doi: 10.3390/ijms242015332. Int J Mol Sci. 2023. PMID: 37895012 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

