Fish Oil and Selenium with Doxorubicin Modulates Expression of Fatty Acid Receptors and Selenoproteins, and Targets Multiple Anti-Cancer Signaling in Triple-negative Breast Cancer Tumors
- PMID: 36483592
- PMCID: PMC9724242
- DOI: 10.7150/ijms.75848
Fish Oil and Selenium with Doxorubicin Modulates Expression of Fatty Acid Receptors and Selenoproteins, and Targets Multiple Anti-Cancer Signaling in Triple-negative Breast Cancer Tumors
Abstract
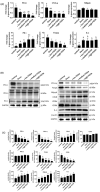
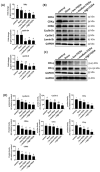
Omega-3 fatty acids from fish oil (FO) and selenium (Se) potentiate some conventional therapies and have anticancer immune potential. This study aims to determine whether FO/Se modulates G-protein-coupled polyunsaturated fatty acid receptors (GPR-40 and GPR-120) and selenoproteins (Sel-H, Sel-W, and GPx4), and increases the therapeutic effect of doxorubicin in a dose-dependent manner on triple-negative breast cancer (TNBC) mouse. Mice were randomized into 5 groups (n = 7/group) and treated with physiological saline (control), low-dose doxorubicin, and doxorubicin in combination with low, medium, or high doses of FO/Se. The expression of signaling molecules in tumors was determined by measuring either mRNA or protein expression. Compared with doxorubicin alone, combination treatment resulted in lower tumor sizes and fewer overall metastasis, lower GPR-40 mRNA levels, and higher expression of all selenoproteins. Doxorubicin-FO/Se combination treatment decreased expression of membrane EGFR and FGFR, down-regulated downstream PI3K/AKT/mTOR, MAPK/ERK, and JAK2/c-Src/STAT3 signaling, increased tumor suppressor PTEN/TSC1/TSC2 expression and P53 activation, and suppressed oncogenic transcription factor expression. Dose-dependent inhibition of proliferation index Ki-67, cell cycle, and stem-cell-related markers were observed. Decreased immune check-points PD-L1/CTLA-4/Foxp3/CD86 and increased PD-1/CD28/IL-2 expression was also found. These observations suggest that the nutritional supplements FO/Se increase the chemotherapeutic efficacy of doxorubicin against TNBC by modulating GPR-40 and selenoprotein and targeting multiple signaling pathways in tumor tissues.
Keywords: Anticancer signaling; Doxorubicin; Fish oil and selenium; GPR-40; Immune microenvironment; Selenoproteins; TNBC mouse tumor.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





References
-
- Marra A, Curigliano G. Adjuvant and neoadjuvant treatment of triple-negative breast cancer with chemotherapy. Cancer J. 2021;27:41–49. - PubMed
-
- Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019;321:288–300. - PubMed
-
- Yun UJ, Lee JH, Shim J, Yoon K, Goh SH, Yi EH, Ye SK, Lee JS, Lee H, Park J. et al. Anti-cancer effect of doxorubicin is mediated by downregulation of HMG-CoA reductase via inhibition of EGFR/Src pathway. Lab Invest. 2019;99:1157–72. - PubMed
-
- Liedtke C, Mazouni C, Hess KR, André F, Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B, Green M. et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–81. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

