Historical evolution of robot-assisted cardiac surgery: a 25-year journey
- PMID: 36483613
- PMCID: PMC9723535
- DOI: 10.21037/acs-2022-rmvs-26
Historical evolution of robot-assisted cardiac surgery: a 25-year journey
Abstract
Many patients and surgeons today favor the least invasive access to an operative site. The adoption of robot-assisted cardiac surgery has been slow, but now has come to fruition. The development of modern surgical robots took surgeons close collaboration with mechanical, electrical, and optical engineers. Moreover, the necessary project funding required entrepreneurs, federal grants, and venture capital. Non-robotic minimally invasive cardiac surgery paved the way to the application of surgical robots by making changes in operative approaches, instruments, visioning modalities, cardiopulmonary perfusion techniques, and especially surgeons' attitudes. In this article, the serial development of robot-assisted cardiac surgery is detailed from the beginning and through clinical application. Included are references to the historical and most recent clinical series that have given us the evidence that robot-assisted cardiac surgery is safe and provides excellent outcomes. To this end, in many institutions these procedures now have become a new standard of care. This evolution reflects Sir Isaac Newton's famous 1676 quote when referring to Rene Descartes, "If have seen further [sic] than others, it is by standing on the shoulders of giants".
Keywords: Cardiac; history; minimally invasive; robotic.
2022 Annals of Cardiothoracic Surgery. All rights reserved.
Conflict of interest statement
Conflicts of Interest: The author declares no conflicts of interest.
Figures


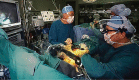


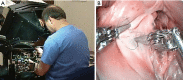

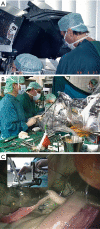





References
-
- Čapek K. Rossumovi Univerzální Roboti (Rossum’s Universal Robots). Prague, 1921.
Publication types
LinkOut - more resources
Full Text Sources
