Antiplatelet efficacy of ticagrelor versus clopidogrel in Mediterranean patients with diabetes mellitus and chronic coronary syndromes: A crossover pharmacodynamic investigation
- PMID: 36483622
- PMCID: PMC9722765
- DOI: 10.3389/fcvm.2022.1057331
Antiplatelet efficacy of ticagrelor versus clopidogrel in Mediterranean patients with diabetes mellitus and chronic coronary syndromes: A crossover pharmacodynamic investigation
Abstract
Introduction: Patients with diabetes mellitus (DM) have augmented platelet reactivity and diminished responsiveness to clopidogrel. Ticagrelor, a more potent P2Y12 inhibitor, is clinically superior to clopidogrel in acute coronary syndromes, although its role in chronic coronary syndromes (CCS) is still the subject of debate. The aim of this investigation was to compare the pharmacodynamic effectiveness of ticagrelor and clopidogrel in Mediterranean DM patients with CCS.
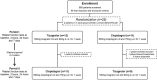
Materials and methods: In this prospective, randomized, crossover study, patients (n = 20) were randomized (1:1) to receive, on top of aspirin therapy, either ticagrelor 180 mg loading dose (LD)/90 mg maintenance dose (MD) b.i.d. or clopidogrel 600 mg LD/75 mg MD o.d. for 1 week in a crossover fashion with a 2-4 week washout period between regimens. Platelet function measurements were performed at 4 timepoints in each period (baseline, 2 h and 24 h after LD, and 1 week), including light transmission aggregometry (LTA, primary endpoint), VASP assay, Multiplate and VerifyNow P2Y12.
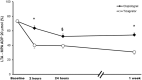
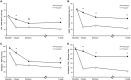
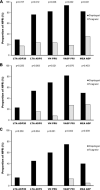
Results: The ticagrelor LD achieved greater platelet inhibitory effect than clopidogrel LD, assessed with LTA (20 μM ADP as agonist), at 2 h (34.9 ± 3.9% vs. 63.6 ± 3.9%; p < 0.001) and 24 h (39.4 ± 3.5% vs. 52.3 ± 3.8%; p = 0.014). After 1 week of therapy, platelet reactivity was again significantly inferior with ticagrelor compared to clopidogrel (30.7 ± 3.0% vs. 54.3 ± 3.0%; p < 0.001). The results were consistent with the other platelet function assays employed.
Conclusion: In Mediterranean patients with DM and CCS, ticagrelor provides a more potent antiplatelet effect than clopidogrel after the LD and during the maintenance phase of therapy.
Clinical trial registration: [ClinicalTrials.gov], identifier [NCT02457130].
Keywords: antiplatelet therapy; chronic coronary syndrome; diabetes mellitus; high platelet reactivity; ticagrelor.
Copyright © 2022 Marcano, Gracida, Roura, Gomez-Lara, Romaguera, Teruel, Fuentes, Muntané-Carol, Meroño, Sosa, Gómez-Hospital, Comin-Colet and Ferreiro.
Conflict of interest statement
JF reports: Speaker fees from Eli Lilly Co., Daiichi Sankyo, Inc., AstraZeneca, Roche Diagnostics, Pfizer, Abbott, Ferrer, Rovi, Boehringer Ingelheim, and Bristol-Myers Squibb; consulting fees from AstraZeneca, Eli Lilly Co., Ferrer, Boston Scientific, Pfizer, Boehringer Ingelheim, Daiichi Sankyo, and Bristol-Myers Squibb; and research grant from AstraZeneca. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Patti G, Cavallari I, Andreotti F, Calabrò P, Cirillo P, Denas G, et al. Prevention of atherothrombotic events in patients with diabetes mellitus: from antithrombotic therapies to new-generation glucose-lowering drugs. Nat Rev Cardiol. (2019) 16:113–30. 10.1038/s41569-018-0080-2 - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

