ALD-R491 regulates vimentin filament stability and solubility, cell contractile force, cell migration speed and directionality
- PMID: 36483676
- PMCID: PMC9723350
- DOI: 10.3389/fcell.2022.926283
ALD-R491 regulates vimentin filament stability and solubility, cell contractile force, cell migration speed and directionality
Abstract
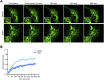
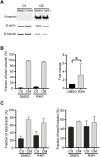
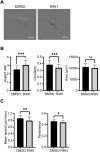
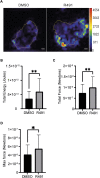
Metastasizing cells express the intermediate filament protein vimentin, which is used to diagnose invasive tumors in the clinic. However, the role of vimentin in cell motility, and if the assembly of non-filamentous variants of vimentin into filaments regulates cell migration remains unclear. We observed that the vimentin-targeting drug ALD-R491 increased the stability of vimentin filaments, by reducing filament assembly and/or disassembly. ALD-R491-treatment also resulted in more bundled and disorganized filaments and an increased pool of non-filamentous vimentin. This was accompanied by a reduction in size of cell-matrix adhesions and increased cellular contractile forces. Moreover, during cell migration, cells showed erratic formation of lamellipodia at the cell periphery, loss of coordinated cell movement, reduced cell migration speed, directionality and an elongated cell shape with long thin extensions at the rear that often detached. Taken together, these results indicate that the stability of vimentin filaments and the soluble pool of vimentin regulate the speed and directionality of cell migration and the capacity of cells to migrate in a mechanically cohesive manner. These observations suggest that the stability of vimentin filaments governs the adhesive, physical and migratory properties of cells, and expands our understanding of vimentin functions in health and disease, including cancer metastasis.
Keywords: cell migration; contractile forces; cytoskeletal organization; fluorescence recovery after photobleaching (FRAP); intermediate filaments (IFs); traction force microscopy (TFM); vimentin; vimentin assembly.
Copyright © 2022 Kim, Warrington, López-Guajardo, Al Hennawi, Cook, Griffith, Symmes, Zhang, Qu, Xu, Chen and Gad.
Conflict of interest statement
Authors DS and RC were employed by Aluda Pharmaceuticals, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Cheng F., Shen Y., Mohanasundaram P., Lindstrom M., Ivaska J., Ny T., et al. (2016). Vimentin coordinates fibroblast proliferation and keratinocyte differentiation in wound healing via TGF-beta-Slug signaling. Proc. Natl. Acad. Sci. U. S. A. 113, E4320–E4327. 10.1073/pnas.1519197113 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

