The Arabidopsis thylakoid chloride channel ClCe regulates ATP availability for light-harvesting complex II protein phosphorylation
- PMID: 36483957
- PMCID: PMC9722747
- DOI: 10.3389/fpls.2022.1050355
The Arabidopsis thylakoid chloride channel ClCe regulates ATP availability for light-harvesting complex II protein phosphorylation
Abstract
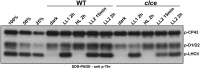
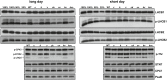
Coping with changes in light intensity is challenging for plants, but well-designed mechanisms allow them to acclimate to most unpredicted situations. The thylakoid K+/H+ antiporter KEA3 and the voltage-dependent Cl- channel VCCN1 play important roles in light acclimation by fine-tuning electron transport and photoprotection. Good evidence exists that the thylakoid Cl- channel ClCe is involved in the regulation of photosynthesis and state transitions in conditions of low light. However, a detailed mechanistic understanding of this effect is lacking. Here we report that the ClCe loss-of-function in Arabidopsis thaliana results in lower levels of phosphorylated light-harvesting complex II (LHCII) proteins as well as lower levels of the photosystem I-LHCII complexes relative to wild type (WT) in low light conditions. The phosphorylation of the photosystem II core D1/D2 proteins was less affected either in low or high light conditions. In low light conditions, the steady-state levels of ATP synthase conductivity and of the total proton flux available for ATP synthesis were lower in ClCe loss-of-function mutants, but comparable to WT at standard and high light intensity. As a long-term acclimation strategy, expression of the ClCe gene was upregulated in WT plants grown in light-limiting conditions, but not in WT plants grown in standard light even when exposed for up to 8 h to low light. Taken together, these results suggest a role of ClCe in the regulation of the ATP synthase activity which under low light conditions impacts LHCII protein phosphorylation and state transitions.
Keywords: ATP synthase; Arabidopsis thaliana; chloride channel (ClC); light-harvesting complex II (LHCII); low light acclimation; photosystem II; protein phosphorylation; proton motive force (PMF).
Copyright © 2022 Dukic, Gollan, Grebe, Paakkarinen, Herdean, Aro and Spetea.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
K+ and Cl- channels/transporters independently fine-tune photosynthesis in plants.Sci Rep. 2019 Jun 14;9(1):8639. doi: 10.1038/s41598-019-44972-z. Sci Rep. 2019. PMID: 31201341 Free PMC article.
-
The Arabidopsis Thylakoid Chloride Channel AtCLCe Functions in Chloride Homeostasis and Regulation of Photosynthetic Electron Transport.Front Plant Sci. 2016 Feb 9;7:115. doi: 10.3389/fpls.2016.00115. eCollection 2016. Front Plant Sci. 2016. PMID: 26904077 Free PMC article.
-
Comparative analysis of thylakoid protein complexes in state transition mutants nsi and stn7: focus on PSI and LHCII.Photosynth Res. 2020 Jul;145(1):15-30. doi: 10.1007/s11120-020-00711-4. Epub 2020 Jan 23. Photosynth Res. 2020. PMID: 31975158 Free PMC article.
-
Contribution of Cyclic and Pseudo-cyclic Electron Transport to the Formation of Proton Motive Force in Chloroplasts.Mol Plant. 2017 Jan 9;10(1):20-29. doi: 10.1016/j.molp.2016.08.004. Epub 2016 Aug 26. Mol Plant. 2017. PMID: 27575692 Review.
-
Thylakoid protein phosphorylation in dynamic regulation of photosystem II in higher plants.Biochim Biophys Acta. 2012 Jan;1817(1):232-8. doi: 10.1016/j.bbabio.2011.05.005. Epub 2011 May 14. Biochim Biophys Acta. 2012. PMID: 21605541 Review.
Cited by
-
Photosynthetic and transcriptome responses to fluctuating light in Arabidopsis thylakoid ion transport triple mutant.Plant Direct. 2023 Oct 25;7(10):e534. doi: 10.1002/pld3.534. eCollection 2023 Oct. Plant Direct. 2023. PMID: 37886682 Free PMC article.
References
-
- Carrillo L. R., Froehlich J. E., Cruz J. A., Savage L. J., Kramer D. M. (2016). Multi-level regulation of the chloroplast ATP synthase: the chloroplast NADPH thioredoxin reductase c (NTRC) is required for redox modulation specifically under low irradiance. Plant J. 87, 654–663. doi: 10.1111/tpj.13226 - DOI - PubMed
-
- Crepin A., Caffarri S. (2015). The specific localizations of phosphorylated Lhcb1 and Lhcb2 isoforms reveal the role of Lhcb2 in the formation of the PSI-LHCII supercomplex in Arabidopsis during state transitions. Biochim. Biophys. Acta 1847, 1539–1548. doi: 10.1016/j.bbabio.2015.09.005 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

