Influence of Helicobacter pylori oncoprotein CagA in gastric cancer: A critical-reflective analysis
- PMID: 36483973
- PMCID: PMC9724182
- DOI: 10.5306/wjco.v13.i11.866
Influence of Helicobacter pylori oncoprotein CagA in gastric cancer: A critical-reflective analysis
Abstract
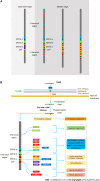
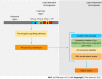
Gastric cancer is the fifth most common malignancy and third leading cancer-related cause of death worldwide. Helicobacter pylori is a Gram-negative bacterium that inhabits the gastric environment of 60.3% of the world's population and represents the main risk factor for the onset of gastric neoplasms. CagA is the most important virulence factor in H. pylori, and is a translocated oncoprotein that induces morphofunctional modifications in gastric epithelial cells and a chronic inflammatory response that increases the risk of developing precancerous lesions. Upon translocation and tyrosine phosphorylation, CagA moves to the cell membrane and acts as a pathological scaffold protein that simultaneously interacts with multiple intracellular signaling pathways, thereby disrupting cell proliferation, differentiation and apoptosis. All these alterations in cell biology increase the risk of damaged cells acquiring pro-oncogenic genetic changes. In this sense, once gastric cancer sets in, its perpetuation is independent of the presence of the oncoprotein, characterizing a "hit-and-run" carcinogenic mechanism. Therefore, this review aims to describe H. pylori- and CagA-related oncogenic mechanisms, to update readers and discuss the novelties and perspectives in this field.
Keywords: CagA; EPIYA motifs; Gastric cancer; Helicobacter pylori; Hit-and-run carcinogenesis; Virulence factors.
©The Author(s) 2022. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Figures

Similar articles
-
Molecular anatomy and pathogenic actions of Helicobacter pylori CagA that underpin gastric carcinogenesis.Cell Mol Immunol. 2020 Jan;17(1):50-63. doi: 10.1038/s41423-019-0339-5. Epub 2019 Dec 5. Cell Mol Immunol. 2020. PMID: 31804619 Free PMC article. Review.
-
Helicobacter pylori CagA oncoprotein interacts with SHIP2 to increase its delivery into gastric epithelial cells.Cancer Sci. 2020 May;111(5):1596-1606. doi: 10.1111/cas.14391. Epub 2020 Apr 13. Cancer Sci. 2020. PMID: 32198795 Free PMC article.
-
Impact of the Helicobacter pylori Oncoprotein CagA in Gastric Carcinogenesis.Curr Top Microbiol Immunol. 2023;444:239-257. doi: 10.1007/978-3-031-47331-9_9. Curr Top Microbiol Immunol. 2023. PMID: 38231221
-
Novel effects of Helicobacter pylori CagA on key genes of gastric cancer signal transduction: a comparative transfection study.Pathog Dis. 2015 Apr;73(3):ftu021. doi: 10.1093/femspd/ftu021. Epub 2014 Dec 12. Pathog Dis. 2015. PMID: 25743471
-
Malignant Helicobacter pylori-Associated Diseases: Gastric Cancer and MALT Lymphoma.Adv Exp Med Biol. 2019;1149:135-149. doi: 10.1007/5584_2019_363. Adv Exp Med Biol. 2019. PMID: 31016622 Review.
Cited by
-
More powerful dysregulation of Helicobacter pylori East Asian-type CagA on intracellular signalings.BMC Microbiol. 2024 Nov 11;24(1):467. doi: 10.1186/s12866-024-03619-4. BMC Microbiol. 2024. PMID: 39528935 Free PMC article.
-
Molecular Mechanisms and Treatment Strategies for Helicobacter pylori-Induced Gastric Carcinogenesis and Mucosa-Associated Lymphoid Tissue (MALT) Lymphoma.Cureus. 2024 May 15;16(5):e60326. doi: 10.7759/cureus.60326. eCollection 2024 May. Cureus. 2024. PMID: 38883131 Free PMC article. Review.
-
Conjugated therapeutic proteins as a treatment for bacteria which trigger cancer development.iScience. 2024 Sep 26;27(10):111029. doi: 10.1016/j.isci.2024.111029. eCollection 2024 Oct 18. iScience. 2024. PMID: 39635133 Free PMC article. Review.
-
New Quipazine Derivatives Active Against Drug-Resistant Oncogenic Helicobacter pylori Strains with Biofilm.Int J Mol Sci. 2025 Jun 22;26(13):5997. doi: 10.3390/ijms26135997. Int J Mol Sci. 2025. PMID: 40649776 Free PMC article.
-
Long-term assessment of Helicobacter pylori cagA EPIYA motif changes and pathology outcomes in gastric biopsies of dyspeptic patients: 10-year follow-up.BMC Gastroenterol. 2024 Dec 19;24(1):466. doi: 10.1186/s12876-024-03516-0. BMC Gastroenterol. 2024. PMID: 39702056 Free PMC article.
References
-
- Warsinggih , Syarifuddin E, Marhamah , Lusikooy RE, Labeda I, Sampetoding S, Dani MI, Kusuma MI, Uwuratuw JA, Prihantono , Faruk M. Association of clinicopathological features and gastric cancer incidence in a single institution. Asian J Surg. 2022;45:246–249. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

