Placental Inflammation Leads to Abnormal Embryonic Heart Development
- PMID: 36484244
- PMCID: PMC10022676
- DOI: 10.1161/CIRCULATIONAHA.122.061934
Placental Inflammation Leads to Abnormal Embryonic Heart Development
Abstract
Background: Placental heart development and embryonic heart development occur in parallel, and these organs have been proposed to exert reciprocal regulation during gestation. Poor placentation has been associated with congenital heart disease, an important cause of infant mortality. However, the mechanisms by which altered placental development can lead to congenital heart disease remain unresolved.
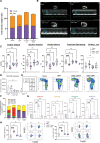
Methods: In this study, we use an in vivo neutrophil-driven placental inflammation model through antibody depletion of maternal circulating neutrophils at key stages during time-mated murine pregnancy: embryonic days 4.5 and 7.5. Pregnant mice were culled at embryonic day 14.5 to assess placental and embryonic heart development. A combination of flow cytometry, histology, and bulk RNA sequencing was used to assess placental immune cell composition and tissue architecture. We also used flow cytometry and single-cell sequencing to assess embryonic cardiac immune cells at embryonic day 14.5 and histology and gene analyses to investigate embryonic heart structure and development. In some cases, offspring were culled at postnatal days 5 and 28 to assess any postnatal cardiac changes in immune cells, structure, and cardiac function, as measured by echocardiography.
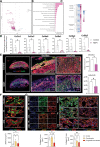
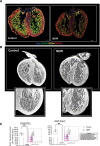
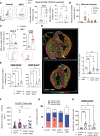
Results: In the present study, we show that neutrophil-driven placental inflammation leads to inadequate placental development and loss of barrier function. Consequently, placental inflammatory monocytes of maternal origin become capable of migration to the embryonic heart and alter the normal composition of resident cardiac macrophages and cardiac tissue structure. This cardiac impairment continues into postnatal life, hindering normal tissue architecture and function. Last, we show that tempering placental inflammation can prevent this fetal cardiac defect and is sufficient to promote normal cardiac function in postnatal life.
Conclusions: Taken together, these observations provide a mechanistic paradigm whereby neutrophil-driven inflammation in pregnancy can preclude normal embryonic heart development as a direct consequence of poor placental development, which has major implications on cardiac function into adult life.
Keywords: fetus; heart defects, congenital; inflammation; macrophages; mothers; neutrophils; placenta.
Conflict of interest statement
None.
Figures


Comment in
-
Don't Break the Axis: Placental Inflammation Leads to Congenital Heart Disease.Circulation. 2023 Mar 21;147(12):973-976. doi: 10.1161/CIRCULATIONAHA.123.063657. Epub 2023 Mar 21. Circulation. 2023. PMID: 36944033 No abstract available.
References
-
- Jorgensen M, McPherson E, Zaleski C, Shivaram P, Cold C. Stillbirth: the heart of the matter. Am J Med Genet A. 2014;164:691–699. doi: 10.1002/ajmg.a.36366 - PubMed
-
- National Congenital Anomaly and Rare Disease Registration Service . NCARDRS congenital anomaly statistics 2017. PHE publications gateway number GW-473. 2017. Accessed December 13, 2022. https://www.gov.uk/government/publications/ncardrs-congenital-anomaly-an...
-
- Doubilet PM, Benson CB. Embryonic heart rate in the early first trimester: what rate is normal? J Ultrasound Med. 1995;14:431–434. doi: 10.7863/jum.1995.14.6.431 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

