G9a inhibition promotes the formation of pacemaker-like cells by reducing the enrichment of H3K9me2 in the HCN4 promoter region
- PMID: 36484369
- PMCID: PMC9813554
- DOI: 10.3892/mmr.2022.12908
G9a inhibition promotes the formation of pacemaker-like cells by reducing the enrichment of H3K9me2 in the HCN4 promoter region
Abstract
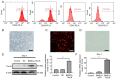
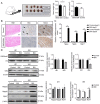
Biological pacemakers, made of pacemaker-like cells, are promising in the treatment of bradyarrhythmia; however, the inefficiency of stem cell differentiation into pacemaker-like cells has limited their clinical application. Previous studies have reported that histone H3 at lysine 9 (H3K9) methylation is widely involved in the proliferation and differentiation of cardiomyocytes, but the specific role of H3K9 dimethylation (H3K9me2) in the formation of pacemaker cells remains unclear. The present study evaluated the functional role of H3K9me2 in the differentiation of bone marrow mesenchymal stem cells (BMSCs) into pacemaker-like cells. Rat BMSCs pretreated with the euchromatic histone lysine methyltransferase 2 (G9a) inhibitor BIX01294 were transfected with a T-box 18 overexpression plasmid to induce BMSCs to form pacemaker-like cells. The induced pacemaker-like cells were analyzed using reverse transcription-quantitative PCR (RT-qPCR) and immunofluorescence to assess the efficiency of differentiation. The enrichment of H3K9me2 in the hyperpolarized-activated cyclic nucleotide-gated cation channel (HCN)4 promoter region was assessed by chromatin immunoprecipitation (ChIP). In addition, BIX01294 was injected into rats, and the protein and mRNA expression levels of HCN4 were assessed using western blotting and RT-qPCR. After interference with G9a using BIX01294, ChIP results demonstrated that H3K9me2 levels in the promoter region of HCN4 were markedly decreased. Immunofluorescence and RT-qPCR demonstrated that the protein expression levels of certain cardio-specific proteins in the treated group were significantly higher compared with those in the untreated group. In vivo experiments demonstrated that interference with G9a could cause pathological hypertrophy. Furthermore, in vitro and in vivo inhibition of G9a could increase the differentiation and proliferation of pacemaker-like cells by decreasing the levels of H3K9me2 in the promoter region of HCN4 gene.
Keywords: euchromatic histone lysine methyltransferase 2; histone H3 at lysine 9; hyperpolarized-activated cyclic nucleotide-gated cation channel 4; pacemaker-like cells; promoter region.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Gorabi AM, Hajighasemi S, Tafti HA, Atashi A, Soleimani M, Aghdami N, Saeid AK, Khori V, Panahi Y, Sahebkar A. TBX18 transcription factor overexpression in human-induced pluripotent stem cells increases their differentiation into pacemaker-like cells. J Cell Physiol. 2019;234:1534–1546. doi: 10.1002/jcp.27018. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

