Safety and Immunogenicity of a ChAd155-Vectored Respiratory Syncytial Virus (RSV) Vaccine in Healthy RSV-Seropositive Children 12-23 Months of Age
- PMID: 36484484
- PMCID: PMC10226655
- DOI: 10.1093/infdis/jiac481
Safety and Immunogenicity of a ChAd155-Vectored Respiratory Syncytial Virus (RSV) Vaccine in Healthy RSV-Seropositive Children 12-23 Months of Age
Abstract
Background: Safe and effective respiratory syncytial virus (RSV) vaccines remain elusive. This was a phase I/II trial (NCT02927873) of ChAd155-RSV, an investigational chimpanzee adenovirus-RSV vaccine expressing 3 proteins (fusion, nucleoprotein, and M2-1), administered to 12-23-month-old RSV-seropositive children followed up for 2 years after vaccination.
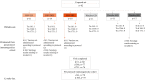
Methods: Children were randomized to receive 2 doses of ChAd155-RSV or placebo (at a 1:1 ratio) (days 1 and 31). Doses escalated from 0.5 × 1010 (low dose [LD]) to 1.5 × 1010 (medium dose [MD]) to 5 × 1010 (high dose [HD]) viral particles after safety assessment. Study end points included anti-RSV-A neutralizing antibody (Nab) titers through year 1 and safety through year 2.
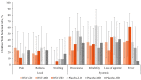
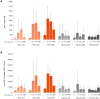
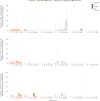
Results: Eighty-two participants were vaccinated, including 11, 14, and 18 in the RSV-LD, RSV-MD, and RSV-HD groups, respectively, and 39 in the placebo groups. Solicited adverse events were similar across groups, except for fever (more frequent with RSV-HD). Most fevers were mild (≤38.5°C). No vaccine-related serious adverse events or RSV-related hospitalizations were reported. There was a dose-dependent increase in RSV-A Nab titers in all groups after dose 1, without further increase after dose 2. RSV-A Nab titers remained higher than prevaccination levels at year 1.
Conclusions: Three ChAd155-RSV dosages were found to be well tolerated. A dose-dependent immune response was observed after dose 1, with no observed booster effect after dose 2. Further investigation of ChAd155-RSV in RSV-seronegative children is warranted.
Clinical trials registration: NCT02927873.
Keywords: immunogenicity; neutralizing antibodies; respiratory syncytial virus; safety; vaccine.
Plain language summary
Respiratory syncytial virus (RSV) is among the main causes of bronchiolitis and pneumonia regularly leading to hospitalization in children. A safe and effective vaccine to prevent RSV infection in this age group has not yet been found, despite great efforts over several decades. This study tested a new candidate RSV vaccine, expressing 3 important pieces of the virus, in toddlers who already had a previous RSV infection. The vaccine was generally well tolerated. Vaccination triggered antibodies against RSV that were able to block the virus in laboratory tests and that persisted for 1 year.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. J. D. D. reports personal fees/grants from GSK, Sanofi Pasteur and MSD and nonfinancial support from Sanofi Pasteur and MSD. X. S. L. reports grants from Cevaxin. Outside the submitted work, C. E. reports support from GSK and ViiV for scientific congress attendance. F. M. T. reports personal fees and nonfinancial support from GSK, Medimmune, Pfizer, MSD, Sanofi Pasteur, Astra Zeneca, and Seqirus. J. M. L. reports grants from GSK and Janssen, personal fees from Pfizer, and nonfinancial support from Immunovaccine. J. B. D. reports consulting fees from Sanofi Pasteur. S. E. reports personal fees and grants from GSK, Sanofi, and Vifor and grants from Abbott and Pfizer. P. M., T. L. A. N., V. N., W. W., Y. Z., I. D., A. L., A. G. L., and N. V. are employees of the GSK group of companies or were employees during the conduct of the study. P. M., W. W., I. D., and A. L. hold shares in the GSK group of companies. The institutions of J. D. D., A. C., A. A. B., F. M. T., J. M. L., J. B. D., and S. E. received funds from GSK for conducting the present work. Outside the submitted work, the institution of F. M. T. received funds from GSK, Ablynx, Jansen, Regeneron, Pfizer, MSD, Novavax, Roche, Astra Zeneca and Seqirus, and the institution of J. B. D. received grants from Merck and Medimmune/Astra Zeneca. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

References
-
- Li Y, Johnson EK, Shi T, et al. . National burden estimates of hospitalisations for acute lower respiratory infections due to respiratory syncytial virus in young children in 2019 among 58 countries: a modelling study. Lancet Respir Med 2021; 9:175–85. - PubMed
-
- Mazur NI, Higgins D, Nunes MC, et al. . The respiratory syncytial virus vaccine landscape: lessons from the graveyard and promising candidates. Lancet Infect Dis 2018; 18:e295–311. - PubMed
-
- Kim HW, Canchola JG, Brandt CD, et al. . Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 1969; 89:422–34. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

