Acid-sensing ion channel 1a activates IKCa/SKCa channels and contributes to endothelium-dependent dilation
- PMID: 36484717
- PMCID: PMC9984545
- DOI: 10.1085/jgp.202213173
Acid-sensing ion channel 1a activates IKCa/SKCa channels and contributes to endothelium-dependent dilation
Abstract
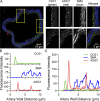
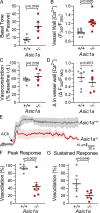
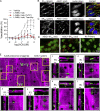
Acid-sensing ion channel 1a (ASIC1a) belongs to a novel family of proton-gated cation channels that are permeable to both Na+ and Ca2+. ASIC1a is expressed in vascular smooth muscle and endothelial cells in a variety of vascular beds, yet little is known regarding the potential impact of ASIC1a to regulate local vascular reactivity. Our previous studies in rat mesenteric arteries suggest ASIC1a does not contribute to agonist-induced vasoconstriction but may mediate a vasodilatory response. The objective of the current study is to determine the role of ASIC1a in systemic vasodilatory responses by testing the hypothesis that the activation of endothelial ASIC1a mediates vasodilation of mesenteric resistance arteries through an endothelium-dependent hyperpolarization (EDH)-related pathway. The selective ASIC1a antagonist psalmotoxin 1 (PcTX1) largely attenuated the sustained vasodilatory response to acetylcholine (ACh) in isolated, pressurized mesenteric resistance arteries and ACh-mediated Ca2+ influx in freshly isolated mesenteric endothelial tubes. Similarly, basal tone was enhanced and ACh-induced vasodilation blunted in mesenteric arteries from Asic1a knockout mice. ASIC1a colocalizes with intermediate- and small-conductance Ca2+-activated K+ channels (IKCa and SKCa, respectively), and the IKCa/SKCa-sensitive component of the ACh-mediated vasodilation was blocked by ASIC1a inhibition. To determine the role of ASIC1a to activate IKCa/SKCa channels, we measured whole-cell K+ currents using the perforated-patch clamp technique in freshly isolated mesenteric endothelial cells. Inhibition of ASIC1a prevented ACh-induced activation of IKCa/SKCa channels. The ASIC1 agonist, α/β-MitTx, activated IKCa/SKCa channels and induced an IKCa/SKCa-dependent vasodilation. Together, the present study demonstrates that ASIC1a couples to IKCa/SKCa channels in mesenteric resistance arteries to mediate endothelium-dependent vasodilation.
© 2022 Garcia et al.
Figures






References
-
- Adapala, R.K., Talasila P.K., Bratz I.N., Zhang D.X., Suzuki M., Meszaros J.G., and Thodeti C.K.. 2011. PKCα mediates acetylcholine-induced activation of TRPV4-dependent calcium influx in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 301:H757–H765. 10.1152/ajpheart.00142.2011 - DOI - PMC - PubMed
-
- Bashari, E., Qadri Y.J., Zhou Z.-H., Kapoor N., Anderson S.J., Meltzer R.H., Fuller C.M., and Benos D.J.. 2009. Two PKC consensus sites on human acid-sensing ion channel 1b differentially regulate its function. Am. J. Physiol. Cell Physiol. 296:C372–C384. 10.1152/ajpcell.00200.2008 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

