LEF1 Drives a Central Memory Program and Supports Antitumor Activity of Natural Killer T Cells
- PMID: 36484736
- PMCID: PMC9898189
- DOI: 10.1158/2326-6066.CIR-22-0333
LEF1 Drives a Central Memory Program and Supports Antitumor Activity of Natural Killer T Cells
Abstract
Vα24-invariant natural killer T cells (NKT) possess innate antitumor properties that can be exploited for cancer immunotherapy. We have shown previously that the CD62L+ central memory-like subset of these cells drives the in vivo antitumor activity of NKTs, but molecular mediators of NKT central memory differentiation remain unknown. Here, we demonstrate that relative to CD62L- cells, CD62L+ NKTs express a higher level of the gene encoding the Wnt/β-catenin transcription factor lymphoid enhancer binding factor 1 (LEF1) and maintain active Wnt/β-catenin signaling. CRISPR/Cas9-mediated LEF1 knockout reduced CD62L+ frequency after antigenic stimulation, whereas Wnt/β-catenin activator Wnt3a ligand increased CD62L+ frequency. LEF1 overexpression promoted NKT expansion and limited exhaustion following serial tumor challenge and was sufficient to induce a central memory-like transcriptional program in NKTs. In mice, NKTs expressing a GD2-specific chimeric-antigen receptor (CAR) with LEF1 demonstrated superior control of neuroblastoma xenograft tumors compared with control CAR-NKTs. These results identify LEF1 as a transcriptional activator of the NKT central memory program and advance development of NKT cell-based immunotherapy. See related Spotlight by Van Kaer, p. 144.
©2022 American Association for Cancer Research.
Figures



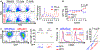

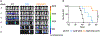
Comment in
-
LEF1 Creates Memories in iNKT Cells That Potentiate Antitumor Immunity.Cancer Immunol Res. 2023 Feb 3;11(2):144. doi: 10.1158/2326-6066.CIR-22-0902. Cancer Immunol Res. 2023. PMID: 36630221
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

