Multiplexed fluidic circuit board for controlled perfusion of 3D blood vessels-on-a-chip
- PMID: 36484766
- PMCID: PMC9764810
- DOI: 10.1039/d2lc00686c
Multiplexed fluidic circuit board for controlled perfusion of 3D blood vessels-on-a-chip
Abstract
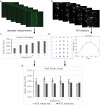
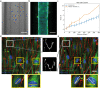
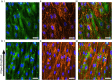
Three-dimensional (3D) blood vessels-on-a-chip (VoC) models integrate the biological complexity of vessel walls with dynamic microenvironmental cues, such as wall shear stress (WSS) and circumferential strain (CS). However, these parameters are difficult to control and are often poorly reproducible due to the high intrinsic diameter variation of individual 3D-VoCs. As a result, the throughput of current 3D systems is one-channel-at-a-time. Here, we developed a fluidic circuit board (FCB) for simultaneous perfusion of up to twelve 3D-VoCs using a single set of control parameters. By designing the internal hydraulic resistances in the FCB appropriately, it was possible to provide a pre-set WSS to all connected 3D-VoCs, despite significant variation in lumen diameters. Using this FCB, we found that variation of CS or WSS induce morphological changes to human induced pluripotent stem cell (hiPSC)-derived endothelial cells (ECs) and conclude that control of these parameters using a FCB is necessary to study 3D-VOCs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Sheinberg D. L. McCarthy D. J. Elwardany O. Bryant J. P. Luther E. Chen S. H. Thompson J. W. Starke R. M. Neurosurgical focus. 2019;47:E3. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

