"Dynamical Docking" of Cyclic Dinuclear Au(I) Bis-N-heterocyclic Complexes Facilitates Their Binding to G-Quadruplexes
- PMID: 36484812
- PMCID: PMC9953335
- DOI: 10.1021/acs.inorgchem.2c03041
"Dynamical Docking" of Cyclic Dinuclear Au(I) Bis-N-heterocyclic Complexes Facilitates Their Binding to G-Quadruplexes
Abstract
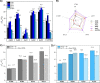
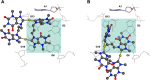
With the aim to improve the design of metal complexes as stabilizers of noncanonical DNA secondary structures, namely, G-quadruplexes (G4s), a series of cyclic dinuclear Au(I) N-heterocyclic carbene complexes based on xanthine and benzimidazole ligands has been synthesized and characterized by various methods, including X-ray diffraction. Fluorescence resonance energy transfer (FRET) and CD DNA melting assays unraveled the compounds' stabilization properties toward G4s of different topologies of physiological relevance. Initial structure-activity relationships have been identified and recognize the family of xanthine derivatives as those more selective toward G4s versus duplex DNA. The binding modes and free-energy landscape of the most active xanthine derivative (featuring a propyl linker) with the promoter sequence cKIT1 have been studied by metadynamics. The atomistic simulations evidenced that the Au(I) compound interacts noncovalently with the top G4 tetrad. The theoretical results on the Au(I) complex/DNA Gibbs free energy of binding were experimentally validated by FRET DNA melting assays. The compounds have also been tested for their antiproliferative properties in human cancer cells in vitro, showing generally moderate activity. This study provides further insights into the biological activity of Au(I) organometallics acting via noncovalent interactions and underlines their promise for tunable targeted applications by appropriate chemical modifications.
Conflict of interest statement
The authors declare no competing financial interest.
Figures









Similar articles
-
Comparative biological evaluation and G-quadruplex interaction studies of two new families of organometallic gold(I) complexes featuring N-heterocyclic carbene and alkynyl ligands.J Inorg Biochem. 2020 Jan;202:110844. doi: 10.1016/j.jinorgbio.2019.110844. Epub 2019 Sep 11. J Inorg Biochem. 2020. PMID: 31739113
-
On the Mechanism of Gold/NHC Compounds Binding to DNA G-Quadruplexes: Combined Metadynamics and Biophysical Methods.Angew Chem Int Ed Engl. 2018 Oct 26;57(44):14524-14528. doi: 10.1002/anie.201805727. Epub 2018 Jul 25. Angew Chem Int Ed Engl. 2018. PMID: 29972613
-
Phenanthroline polyazamacrocycles as G-quadruplex DNA binders.Org Biomol Chem. 2018 Apr 18;16(15):2776-2786. doi: 10.1039/c8ob00247a. Org Biomol Chem. 2018. PMID: 29611599
-
Structurally diverse G-quadruplexes as the noncanonical nucleic acid drug target for live cell imaging and antibacterial study.Chem Commun (Camb). 2023 Feb 2;59(11):1415-1433. doi: 10.1039/d2cc05945b. Chem Commun (Camb). 2023. PMID: 36636928 Review.
-
Metal-Organic Compounds as Anticancer Agents: Versatile Building Blocks for Selective Action on G-quadruplexes.Curr Med Chem. 2023;30(5):573-600. doi: 10.2174/0929867329666220606160209. Curr Med Chem. 2023. PMID: 35670351 Review.
Cited by
-
Harnessing Organometallic Au(III) Complexes as Precision Scaffolds for Next-Generation Therapeutic and Imaging Agents.Chembiochem. 2025 Jul 18;26(14):e202500347. doi: 10.1002/cbic.202500347. Epub 2025 Jun 4. Chembiochem. 2025. PMID: 40341705 Free PMC article. Review.
-
Water-Mediated Chiral Resolution of Ag-NHC(Nucleobase) Complexes.Inorg Chem. 2025 Mar 24;64(11):5487-5494. doi: 10.1021/acs.inorgchem.4c05384. Epub 2025 Feb 10. Inorg Chem. 2025. PMID: 39927891 Free PMC article.
-
Unraveling the Molecular Basis for G-Quadruplex-Binders to ALS/FTD-Associated G4C2 Repeats of the C9orf72 Gene.Chembiochem. 2025 Apr 14;26(8):e202400974. doi: 10.1002/cbic.202400974. Epub 2025 Jan 20. Chembiochem. 2025. PMID: 39670345 Free PMC article.
References
-
- Harbut M. B.; Vilchèze C.; Luo X.; Hensler M. E.; Guo H.; Yang B.; Chatterjee A. K.; Nizet V.; Jacobs W. R.; Schultz P. G.; Wang F. Auranofin Exerts Broad-Spectrum Bactericidal Activities by Targeting Thiol-Redox Homeostasis. Proc. Natl. Acad. Sci. U.S.A. 2015, 112, 4453–4458. 10.1073/pnas.1504022112. - DOI - PMC - PubMed
-
- Koch R. Ueber Bacteriologische Forschung. Dtsch. Med. Wochenschr. 1890, 16, 756–757.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

