Tumor-Infiltrating Lymphocyte Therapy in Melanoma: Facts to the Future
- PMID: 36485001
- PMCID: PMC10183807
- DOI: 10.1158/1078-0432.CCR-22-1922
Tumor-Infiltrating Lymphocyte Therapy in Melanoma: Facts to the Future
Abstract
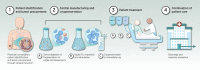
Adoptive cell therapy with tumor-infiltrating lymphocytes (TIL) is gaining momentum and demonstrating durable responses in patients with advanced melanoma. Although increasingly considered as a treatment option for select patients with melanoma, TIL therapy is not yet approved by any regulatory agency. Pioneering studies with first-generation TIL therapy, undertaken before the advent of modern melanoma therapeutics, demonstrated clinical efficacy and remarkable long-term overall survival, reaching beyond 20 months for responding patients. TIL therapy is a multistep process of harvesting patient-specific tumor-resident T cells from tumors, ex vivo T-cell expansion, and re-infusion into the same patient after a lymphodepleting preparative regimen, with subsequent supportive IL2 administration. Objective response rates between 30% and 50% have consistently been observed in heavily pretreated patients with metastatic melanoma, including those who have progressed after modern immune checkpoint inhibitors and BRAF targeted agents, a population with high unmet medical need. Although significant strides have been made in modern TIL therapeutics, refinement strategies to optimize patient selection, enhance TIL production, and improve efficacy are being explored. Here, we review past and present experience, current challenges, practical considerations, and future aspirations in the evolution of TIL therapy for the treatment of melanoma as well as other solid tumors.
©2022 The Authors; Published by the American Association for Cancer Research.
Figures

References
-
- Dummer R, Flaherty K, Robert C, Arance AM, JWd G, Garbe C, et al. . Five-year overall survival (OS) in COLUMBUS: a randomized phase 3 trial of encorafenib plus binimetinib versus vemurafenib or encorafenib in patients (pts) with BRAF V600-mutant melanoma. J Clin Oncol 39:15s, 2021: (suppl. abstr. 9507). - PMC - PubMed
-
- Ugurel S, Röhmel J, Ascierto PA, Becker JC, Flaherty KT, Grob JJ, et al. . Survival of patients with advanced metastatic melanoma: the impact of MAP kinase pathway inhibition and immune checkpoint inhibition-Update 2019. Eur J Cancer 2020;130:126–38. - PubMed
-
- Curti BD, Faries MB. Recent advances in the treatment of melanoma. N Engl J Med 2021;384:2229–40. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

