Leukemogenesis in infants and young children with trisomy 21
- PMID: 36485097
- PMCID: PMC9820574
- DOI: 10.1182/hematology.2022000395
Leukemogenesis in infants and young children with trisomy 21
Abstract
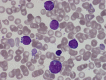
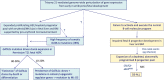
Children with Down syndrome (DS) have a greater than 100-fold increased risk of developing acute myeloid leukemia (ML) and an approximately 30-fold increased risk of acute lymphoblastic leukemia (ALL) before their fifth birthday. ML-DS originates in utero and typically presents with a self-limiting, neonatal leukemic syndrome known as transient abnormal myelopoiesis (TAM) that is caused by cooperation between trisomy 21-associated abnormalities of fetal hematopoiesis and somatic N-terminal mutations in the transcription factor GATA1. Around 10% of neonates with DS have clinical signs of TAM, although the frequency of hematologically silent GATA1 mutations in DS neonates is much higher (~25%). While most cases of TAM/silent TAM resolve without treatment within 3 to 4 months, in 10% to 20% of cases transformation to full-blown leukemia occurs within the first 4 years of life when cells harboring GATA1 mutations persist and acquire secondary mutations, most often in cohesin genes. By contrast, DS-ALL, which is almost always B-lineage, presents after the first few months of life and is characterized by a high frequency of rearrangement of the CRLF2 gene (60%), often co-occurring with activating mutations in JAK2 or RAS genes. While treatment of ML-DS achieves long-term survival in approximately 90% of children, the outcome of DS-ALL is inferior to ALL in children without DS. Ongoing studies in primary cells and model systems indicate that the role of trisomy 21 in DS leukemogenesis is complex and cell context dependent but show promise in improving management and the treatment of relapse, in which the outcome of both ML-DS and DS-ALL remains poor.
Copyright © 2022 by The American Society of Hematology.
Conflict of interest statement
Irene Roberts: no competing financial interests to declare.
Figures


References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

