Hitting the brakes on accelerated and blast-phase myeloproliferative neoplasms: current and emerging concepts
- PMID: 36485103
- PMCID: PMC9820986
- DOI: 10.1182/hematology.2022000341
Hitting the brakes on accelerated and blast-phase myeloproliferative neoplasms: current and emerging concepts
Abstract
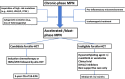
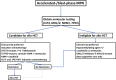
The BCR-ABL-negative myeloproliferative neoplasms (MPNs) have a variable risk of progressing to accelerated- or blast-phase MPN (MPN-AP/MPN-BP), defined by the presence of 10% to 19% and more than or equal to 20% myeloid blasts in the peripheral blood or bone marrow, respectively. The molecular processes underlying the progression to MPN-AP/MPN-BP are becoming increasingly understood with the acquisition of additional mutations in epigenetic modifiers (eg, ASXL1, EZH2, TET2), TP53, the Ras pathway, or splicing factors (eg, SRSF2, U2AF1), having been described as important steps in this evolutionary process. At least partially driven by the enrichment of these high-risk molecular features, the prognosis of patients with MPN-BP remains inferior to other patients with acute myeloid leukemia, with a median overall survival of 3 to 6 months. Allogeneic hematopoietic cell transplantation remains the only potentially curative therapeutic modality, but only a minority of patients are eligible. In the absence of curative intent, therapeutic strategies or palliative treatment with hypomethylating agents as monotherapy or in combination with ruxolitinib or venetoclax can be considered. Several novel agents are in various stages of clinical development but are not available for routine use at this point, highlighting the need for ongoing research and the prioritization of clinical trial enrollment when feasible.
Copyright © 2022 by The American Society of Hematology.
Conflict of interest statement
Jan Philipp Bewersdorf: no competing financial interests to declare.
Raajit K. Rampal: research funding: Constellation, Incyte, Stemline, Zentalis; consultancy: Abbvie, Blueprint, Celgene/Bristol Myers Squibb, Constellation, CTI, Disc Medicines, Galecto, Incyte, Jazz, Novartis, Pharmaessentia, Promedior, Sierra Oncology, Stemline, Sumitomo Pharma, Zentalis.
Figures
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

