Warm autoimmune hemolytic anemia and the best treatment strategies
- PMID: 36485114
- PMCID: PMC9821065
- DOI: 10.1182/hematology.2022000405
Warm autoimmune hemolytic anemia and the best treatment strategies
Abstract
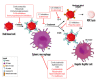
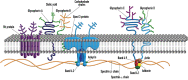
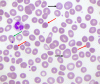
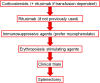
Warm autoimmune hemolytic anemia (wAIHA) is characterized by evidence of red blood cell (RBC) hemolysis and a direct antiglobulin test positive for IgG and sometimes complement. While varying with the extent of the compensatory increase in RBC production, symptoms of anemia predominate, as does jaundice, the latter often exacerbated by concurrent Gilbert's syndrome. Initial treatment with corticosteroids is highly effective, with over 85% of patients responding but with less than one-third maintaining that response upon weaning. Subsequent rituximab administration in those failing corticosteroids provides complete remission in over 75% of patients and may be long-lasting. Over 50% of patients failing rituximab respond to erythropoiesis-stimulating agents or immunosuppressive agents. Splenectomy is best deferred if possible but does offer long-term remission in over two-thirds of patients. A number of new treatments for wAIHA (fostamatinib, rilzabrutinib, and FcRn inhibitors) show promise. A treatment algorithm for wAIHA is proposed to avoid the excessive use of corticosteroids.
Copyright © 2022 by The American Society of Hematology.
Conflict of interest statement
David J. Kuter: research funding: Argenx, Biocryst, Immunovant, Rigel, Sanofi (Principia), Takeda (Bioverativ), UCB; consultancy: Alexion (Syntimmune), Amgen, Argenx, BioCryst, Bristol Myers Squibb, Caremark, Cellphire, Cellularity, CRICO, Daiichi Sankyo, Hengrui, Immunovant, Incyte, Kyowa-Kirin, Merck Sharp Dohme, Momenta, Novartis, Pfizer, Platelet Biogenesis, Platelet Disorder Support Association, Rigel, Sanofi (Bioveratif), Sanofi (Genzyme), Sanofi (Principia), Sobi (Dova), Takeda, UCB, Up-To-Date; stock ownership: Rubius.
Figures

Similar articles
-
[Autoimmune hemolytic anemia].Vnitr Lek. 2018 Summer;64(5):514-519. Vnitr Lek. 2018. PMID: 30193520 Czech.
-
Emergence of long-lived autoreactive plasma cells in the spleen of primary warm auto-immune hemolytic anemia patients treated with rituximab.J Autoimmun. 2015 Aug;62:22-30. doi: 10.1016/j.jaut.2015.05.006. Epub 2015 Jun 23. J Autoimmun. 2015. PMID: 26112660
-
Updates in the Management of Warm Autoimmune Hemolytic Anemia.Hematol Oncol Clin North Am. 2022 Apr;36(2):325-339. doi: 10.1016/j.hoc.2021.11.005. Epub 2022 Mar 11. Hematol Oncol Clin North Am. 2022. PMID: 35282949 Review.
-
A Retrospective Study of the Combination of Rituximab, Cyclophosphamide and Dexamethasone for the Treatment of Relapsed/Refractory Warm Antibody Autoimmune Hemolytic Anemia.Acta Haematol. 2020;143(3):244-249. doi: 10.1159/000501538. Epub 2019 Oct 30. Acta Haematol. 2020. PMID: 31665725
-
Immunotherapy treatments of warm autoimmune hemolytic anemia.Clin Dev Immunol. 2013;2013:561852. doi: 10.1155/2013/561852. Epub 2013 Sep 11. Clin Dev Immunol. 2013. PMID: 24106518 Free PMC article. Review.
Cited by
-
The Role of the Spleen and the Place of Splenectomy in Autoimmune Hemolytic Anemia-A Review of Current Knowledge.Diagnostics (Basel). 2023 Sep 9;13(18):2891. doi: 10.3390/diagnostics13182891. Diagnostics (Basel). 2023. PMID: 37761258 Free PMC article. Review.
-
The choice of new treatments in autoimmune hemolytic anemia: how to pick from the basket?Front Immunol. 2023 Apr 24;14:1180509. doi: 10.3389/fimmu.2023.1180509. eCollection 2023. Front Immunol. 2023. PMID: 37168855 Free PMC article. Review.
-
Autoimmune Hemolytic Anemias: Classifications, Pathophysiology, Diagnoses and Management.Int J Mol Sci. 2024 Apr 12;25(8):4296. doi: 10.3390/ijms25084296. Int J Mol Sci. 2024. PMID: 38673882 Free PMC article. Review.
-
Successful Management of Severe and Refractory Autoimmune Hemolytic Anemia (AIHA) in a Sickle Cell Disease Patient With Bortezomib.Cureus. 2024 Nov 30;16(11):e74840. doi: 10.7759/cureus.74840. eCollection 2024 Nov. Cureus. 2024. PMID: 39737253 Free PMC article.
-
Autoimmune Hemolytic Anemia Associated With Myelodysplastic Syndrome: A Case Report.J Investig Med High Impact Case Rep. 2024 Jan-Dec;12:23247096241273215. doi: 10.1177/23247096241273215. J Investig Med High Impact Case Rep. 2024. PMID: 39171743 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

