Updates in infant acute lymphoblastic leukemia and the potential for targeted therapy
- PMID: 36485124
- PMCID: PMC9821252
- DOI: 10.1182/hematology.2022000359
Updates in infant acute lymphoblastic leukemia and the potential for targeted therapy
Abstract
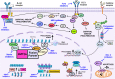
Outcomes for infants diagnosed under 1 year of age with KMT2A-rearranged acute lymphoblastic leukemia (ALL) have remained stagnant over the past 20 years. Successive treatment protocols have previously focused on intensification of conventional chemotherapy, but increased treatment-related toxicity and chemoresistance have led to a plateau in survival. We have now entered an era of immunotherapy with integration of agents, such as blinatumomab or chimeric antigen receptor T-cell therapy, into the standard chemotherapy backbone, showing significant promise for improving the dismal outcomes for this disease. There remains much optimism for the future as a wealth of preclinical studies have identified additional novel targeted agents, such as venetoclax or menin inhibitors, ready for incorporation into treatment, providing further ammunition to combat this aggressive disease. In contrast, infants with KMT2A-germline ALL have demonstrated excellent survival outcomes with current therapy, but there remains a high burden of treatment-related morbidity. Greater understanding of the underlying blast genetics for infants with KMT2A-germline ALL and incorporation of immunotherapeutic approaches may enable a reduction in the intensity of chemotherapy while maintaining the excellent outcomes.
Copyright © 2022 by The American Society of Hematology.
Conflict of interest statement
Rishi S. Kotecha has participated in Scientific Advisory Boards for Amgen, Link Healthcare, and Jazz Pharmaceuticals.
Figures
References
-
- Breese EH, Kotecha RS, Guest EM. Acute lymphoblastic leukemia in infants: a distinctive, high-risk subtype of childhood acute lymphoblastic leukemia. In: Litzow MR, Raetz EA, eds. Clinical Management of Acute Lymphoblastic Leukemia: From Bench to Bedside. Cham, Switzerland: Springer Nature; 2022:135-148.
-
- Guest EM, Kairalla JA, Hilden JM, et al.. Outstanding outcomes in infants with KMT2A-germline acute lymphoblastic leukemia treated with chemotherapy alone: results of the Children's Oncology Group AALL0631 trial. Haematologica. 2022;107(5):1205-1208. doi:10.3324/haematol.2021.280146. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous