Dyskeratosis congenita and telomere biology disorders
- PMID: 36485133
- PMCID: PMC9821046
- DOI: 10.1182/hematology.2022000394
Dyskeratosis congenita and telomere biology disorders
Abstract
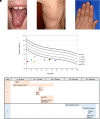
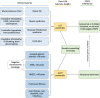
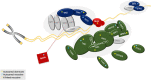
Numerous genetic discoveries and the advent of clinical telomere length testing have led to the recognition of a spectrum of telomere biology disorders (TBDs) beyond the classic dyskeratosis congenita (DC) triad of nail dysplasia, abnormal skin pigmentation, and oral leukoplakia occurring with pediatric bone marrow failure. Patients with DC/TBDs have very short telomeres for their age and are at high risk of bone marrow failure, cancer, pulmonary fibrosis (PF), pulmonary arteriovenous malformations, liver disease, stenosis of the urethra, esophagus, and/or lacrimal ducts, avascular necrosis of the hips and/or shoulders, and other medical problems. However, many patients with TBDs do not develop classic DC features; they may present in middle age and/or with just 1 feature, such as PF or aplastic anemia. TBD-associated clinical manifestations are progressive and attributed to aberrant telomere biology caused by the X-linked recessive, autosomal dominant, autosomal recessive, or de novo occurrence of pathogenic germline variants in at least 18 different genes. This review describes the genetics and clinical manifestations of TBDs and highlights areas in need of additional clinical and basic science research.
Conflict of interest statement
Sharon A. Savage: no competing financial interests to declare.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

