Antibodies and bispecifics for multiple myeloma: effective effector therapy
- PMID: 36485135
- PMCID: PMC9820318
- DOI: 10.1182/hematology.2022000334
Antibodies and bispecifics for multiple myeloma: effective effector therapy
Abstract
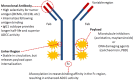
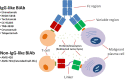
The therapeutic landscape in multiple myeloma (MM) has changed dramatically over the last 2 decades. With the introduction of novel immunotherapies, patients with MM can expect deeper responses, longer remissions, and improved overall survival. Since its approval by the US Food and Drug Administration in 2015, the monoclonal antibody specific for CD38, daratumumab, has been incorporated into both frontline and relapsed treatment regimens. Its role as a maintenance therapy is currently being explored. Subsequently, a variety of novel antibody therapeutics have evolved from the success of daratumumab, using similar concepts to target the malignant plasma cell clone. Noteworthy naked monoclonal antibodies include isatuximab, another agent directed against CD38, and elotuzumab, an agent directed against SLAM family member 7. Antibody-drug conjugates, complex molecules composed of an antibody tethered to a cytotoxic drug, target malignant cells and deliver a lethal payload. The first to market is belantamab mafodotin, which targets B-cell maturation antigen (BCMA) on malignant plasma cells and delivers a potent microtubule inhibitor, monomethyl auristatin F. Additionally, bispecific T-cell antibodies are in development that engage the immune system directly by simultaneously binding CD3 on T cells and a target epitope-such as BCMA, G-protein coupled receptor family C group 5 member D (GPRC5d), and Fc receptor homologue 5 (FcRH5)-on malignant cells. Currently, teclistamab, an anti-BCMA bispecific, is closest to approval for commercial use. In this review, we explore the evolving landscape of antibodies in the treatment of MM, including their role in frontline and relapse settings.
Copyright © 2022 by The American Society of Hematology.
Conflict of interest statement
Christopher Cipkar: no competing financial interests to declare.
Christine Chen: consultancy: Forus Therapeutics; advisory board: Amgen, Bristol Myers Squibb, Janssen Pharmaceuticals.
Suzanne Trudel: research funding: Amgen, Bristol Myers Squibb, Genentech, GlaxoSmithKline, Janssen Pharmaceuticals, Pfizer, Roche; consultancy: Bristol Myers Squibb, Forus, GlaxoSmithKline, K36 Therapeutics, Roche; advisory board: Amgen, Bristol Myers Squibb, GlaxoSmithKline, Janssen Pharmaceuticals, Pfizer, Sanofi.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

