Stable and durable factor IX levels in patients with hemophilia B over 3 years after etranacogene dezaparvovec gene therapy
- PMID: 36490302
- PMCID: PMC10539871
- DOI: 10.1182/bloodadvances.2022008886
Stable and durable factor IX levels in patients with hemophilia B over 3 years after etranacogene dezaparvovec gene therapy
Abstract
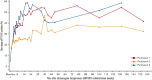
Etranacogene dezaparvovec (AMT-061) is a recombinant adeno-associated virus serotype 5 (AAV5) vector containing a codon-optimized Padua variant human factor IX (FIX) transgene with a liver-specific promoter. Here, we report 3-year outcomes from a phase 2b, open-label, single-dose, single-arm, multicenter trial conducted among adults with severe or moderately severe hemophilia B (FIX ≤2%). All participants (n = 3) received a single intravenous dose (2 × 1013 gene copies per kg) and will be followed up for 5 years. The primary end point of FIX activity ≥5% at 6 weeks was met. Secondary end points included bleed frequency, FIX concentrate use, joint health, and adverse events (AEs). All participants required routine FIX prophylaxis and had neutralizing antibodies to AAV5 before etranacogene dezaparvovec treatment. After administration, FIX activity rose to a mean of 40.8% in year 1 and was sustained in year 3 at 36.9%. All participants discontinued FIX prophylaxis. Bleeding was completely eliminated in 2 out of 3 participants. One participant required on-demand FIX replacement therapy per protocol because of elective surgical procedures, for 2 reported bleeding episodes, and twice for a single self-administered infusion because of an unreported reason. One participant experienced 2 mild, self-limiting AEs shortly after dosing. During the 3-year study period, there were no clinically significant elevations in liver enzymes, no requirement for steroids, no FIX inhibitor development, and no late-emergent safety events in any participant. Etranacogene dezaparvovec was safe and effective in adults with hemophilia B over 3 years after administration. This trial was registered at www.clinicaltrials.gov as #NCT03489291.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: A.v.D. is a consultant for BioMarin, Sanofi Genzyme, Novo Nordisk, Pfizer, uniQure, and Hematherix. A.G. is a consultant for Bioverativ, Genentech/Roche, BioMarin, and uniQure and serves as a speaker bureau of Bioverativ and Genentech/Roche. G.C. received grants/research support from CSL Behring, Pfizer, and Sobi and serves as a speaker bureau of Bayer, BioMarin, Roche, Sobi, Grifols, LFB, Novo Nordisk, Werfen, Kedrion, and uniQure. N.S.K. received grants/research support from Grifols and Takeda and is a consultant for uniQure, BioMarin, and Novo Nordisk. S.U.L is a consultant for uniQure. F.W.G.L. received grants/research support from CSL Behring, Shire/Takeda, Roche, and Sobi and is a consultant for uniQure, Takeda, Novo Nordisk, and BioMarin. W.A.M. received grants/research support from Bayer, Biotest, Takeda, LFB, Octapharma, Novo Nordisk, Pfizer, and uniQure and is a consultant for Bayer, BioMarin, Freeline, LFB, Octapharma, Novo Nordisk, Pfizer, and uniQure. M.R. received research funding from Bayer, BioMarin, CSL Behring, Genentech, Grifols, Hema Biologics, LFB, Novo Nordisk, Octapharma, Pfizer, Sanofi, Spark Therapeutics, Takeda, and uniQure; is a consultant for Catalyst Biosciences, CSL Behring, Genentech, Hema Biologics, Kedrion, Novo Nordisk, Pfizer, Sanofi, Takeda, and uniQure; and is on the board of directors for Foundation for Women and Girls with Blood Disorders, Partners in Bleeding Disorders Education, and Thrombosis and Hemostasis Societies of North America. E.G. serves as a speaker bureau of Global Blood Therapeutics. R.G. is an employee of uniQure. R.D. is an employee of uniQure. S.L.Q. is an employee of CSL Behring. P.E.M. is an employee of CSL Behring. S.W.P. received a grant/research support from Bayer, BioMarin, Freeline, Novo Nordisk, and Roche/Genentech and is a consultant for ApcinteX, ASC Therapeutics, Bayer, BioMarin, CSL Behring, GeneVentiv, HEMA Biologics, Freeline Therapeutics, Novo Nordisk, Pfizer, Regeneron/Intellia, Roche/Genentech, Sanofi, Spark Therapeutics, Takeda, and uniQure.
Figures


References
-
- Srivastava A, Santagostino E, Dougall A, et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020;26(suppl 6):1–158. - PubMed
-
- Young G, Collins PW, Colberg T, et al. Nonacog beta pegol (N9-GP) in haemophilia B: a multinational phase III safety and efficacy extension trial (paradigm™4) Thromb Res. 2016;141:69–76. - PubMed
-
- White GC, Rosendaal F, Aledort LM, et al. Definitions in hemophilia. Recommendation of the scientific subcommittee on factor VIII and factor IX of the scientific and standardization committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost. 2001;85(3):560. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

