Impact of the COVID-19 Vaccination Program on case incidence, emergency department visits, and hospital admissions among children aged 5-17 Years during the Delta and Omicron Periods-United States, December 2020 to April 2022
- PMID: 36490304
- PMCID: PMC9733849
- DOI: 10.1371/journal.pone.0276409
Impact of the COVID-19 Vaccination Program on case incidence, emergency department visits, and hospital admissions among children aged 5-17 Years during the Delta and Omicron Periods-United States, December 2020 to April 2022
Abstract
Background: In the United States, national ecological studies suggest a positive impact of COVID-19 vaccination coverage on outcomes in adults. However, the national impact of the vaccination program on COVID-19 in children remains unknown. To determine the association of COVID-19 vaccination with U.S. case incidence, emergency department visits, and hospital admissions for pediatric populations during the Delta and Omicron periods.
Methods: We conducted an ecological analysis among children aged 5-17 and compared incidence rate ratios (RRs) of COVID-19 cases, emergency department visits, and hospital admissions by pediatric vaccine coverage, with jurisdictions in the highest vaccine coverage quartile as the reference.
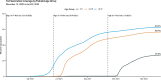
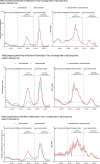
Results: RRs comparing states with lowest pediatric vaccination coverage to the highest pediatric vaccination coverage were 2.00 and 0.64 for cases, 2.96 and 1.11 for emergency department visits, and 2.76 and 1.01 for hospital admissions among all children during the Delta and Omicron periods, respectively. During the 3-week peak period of the Omicron wave, only children aged 12-15 and 16-17 years in the states with the lowest versus highest coverage, had a significantly higher rate of emergency department visits (RR = 1.39 and RR = 1.34, respectively).
Conclusions: COVID-19 vaccines were associated with lower case incidence, emergency department visits and hospital admissions among children during the Delta period but the association was weaker during the Omicron period. Pediatric COVID-19 vaccination should be promoted as part of a program to decrease COVID-19 impact among children; however, vaccine effectiveness may be limited when available vaccines do not match circulating viral variants.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Oliver SE, Gargano JW, Marin M, Wallace M, Curran KG, Chamberland M, et al.. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine—United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1922–1924. doi: 10.15585/mmwr.mm6950e2 - DOI - PMC - PubMed
-
- Wallace M, Woodworth KR, Gargano JW, Scobie HM, Blain AE, Moulia D, et al.. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine in adolescents aged 12–15 years—United States, May 2021. MMWR Morb Mortal Wkly Rep. 2021;70:749–752. doi: 10.15585/mmwr.mm7020e1 - DOI - PMC - PubMed
-
- Woodworth KR, Moulia D, Collins JP, Hadler SC, Jones JM, Reddy SC, et al.. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine in children aged 5–11 years—United States, November 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1579–1583. doi: 10.15585/mmwr.mm7045e1 - DOI - PMC - PubMed
-
- Trends in demographic characteristics of people receiving COVID-19 vaccinations in the United States. US Centers for Disease Control and Prevention. Accessed May 24, 2022. https://covid.cdc.gov/covid-data-tracker/#vaccination-demographic-trends.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

