Oncogenic deubiquitination controls tyrosine kinase signaling and therapy response in acute lymphoblastic leukemia
- PMID: 36490346
- PMCID: PMC9733937
- DOI: 10.1126/sciadv.abq8437
Oncogenic deubiquitination controls tyrosine kinase signaling and therapy response in acute lymphoblastic leukemia
Abstract
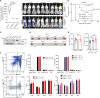
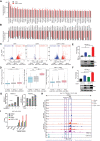
Dysregulation of kinase signaling pathways favors tumor cell survival and therapy resistance in cancer. Here, we reveal a posttranslational regulation of kinase signaling and nuclear receptor activity via deubiquitination in T cell acute lymphoblastic leukemia (T-ALL). We observed that the ubiquitin-specific protease 11 (USP11) is highly expressed and associates with poor prognosis in T-ALL. USP11 ablation inhibits leukemia progression in vivo, sparing normal hematopoiesis. USP11 forms a complex with USP7 to deubiquitinate the oncogenic lymphocyte cell-specific protein-tyrosine kinase (LCK) and enhance its activity. Impairment of LCK activity leads to increased glucocorticoid receptor (GR) expression and glucocorticoids sensitivity. Genetic knockout of USP7 improved the antileukemic efficacy of glucocorticoids in vivo. The transcriptional activation of GR target genes is orchestrated by the deubiquitinase activity and mediated via an increase in enhancer-promoter interaction intensity. Our data unveil how dysregulated deubiquitination controls leukemia survival and drug resistance, suggesting previously unidentified therapeutic combinations toward targeting leukemia.
Figures





Similar articles
-
Therapeutic targeting of LCK tyrosine kinase and mTOR signaling in T-cell acute lymphoblastic leukemia.Blood. 2022 Oct 27;140(17):1891-1906. doi: 10.1182/blood.2021015106. Blood. 2022. PMID: 35544598 Free PMC article.
-
Design, synthesis, and pharmacological evaluation of novel benzothiazole derivatives targeting LCK in acute lymphoblastic leukemia.Bioorg Chem. 2024 Mar;144:107180. doi: 10.1016/j.bioorg.2024.107180. Epub 2024 Feb 5. Bioorg Chem. 2024. PMID: 38335758
-
Use of recombinant cell-permeable small peptides to modulate glucocorticoid sensitivity of acute lymphoblastic leukemia cells.Biochemistry. 2010 Oct 19;49(41):8892-901. doi: 10.1021/bi1007723. Biochemistry. 2010. PMID: 20831260 Free PMC article.
-
New insights into Notch1 regulation of the PI3K-AKT-mTOR1 signaling axis: targeted therapy of γ-secretase inhibitor resistant T-cell acute lymphoblastic leukemia.Cell Signal. 2014 Jan;26(1):149-61. doi: 10.1016/j.cellsig.2013.09.021. Epub 2013 Oct 16. Cell Signal. 2014. PMID: 24140475 Review.
-
Glucocorticoid resistance in childhood leukemia.Leuk Lymphoma. 1994 Apr;13(3-4):187-201. doi: 10.3109/10428199409056282. Leuk Lymphoma. 1994. PMID: 8049644 Review.
Cited by
-
NOTCH1 signaling is dysregulated by loss of the deubiquitinase USP28 with del(11q), uncovering USP28 inhibition as novel therapeutic target in CLL.Leukemia. 2025 Aug;39(8):1892-1904. doi: 10.1038/s41375-025-02632-4. Epub 2025 Jun 2. Leukemia. 2025. PMID: 40456839 Free PMC article.
-
The USP11/TCEAL1 complex promotes transcription elongation to sustain oncogenic gene expression in neuroblastoma.Genes Dev. 2025 Jun 2;39(11-12):751-769. doi: 10.1101/gad.352166.124. Genes Dev. 2025. PMID: 40250979
-
Emerging Epigenetic and Posttranslational Mechanisms Controlling Resistance to Glucocorticoids in Acute Lymphoblastic Leukemia.Hemasphere. 2023 Jun 22;7(7):e916. doi: 10.1097/HS9.0000000000000916. eCollection 2023 Jul. Hemasphere. 2023. PMID: 37359189 Free PMC article. Review.
-
A positive feedback loop regulation between NOTCH1 and USP11 in T-cell leukemia.Leukemia. 2024 Jan;38(1):193-197. doi: 10.1038/s41375-023-02096-4. Epub 2023 Nov 25. Leukemia. 2024. PMID: 38007584 Free PMC article. No abstract available.
-
Beyond Corticoresistance, A Paradoxical Corticosensitivity Induced by Corticosteroid Therapy in Pediatric Acute Lymphoblastic Leukemias.Cancers (Basel). 2023 May 18;15(10):2812. doi: 10.3390/cancers15102812. Cancers (Basel). 2023. PMID: 37345151 Free PMC article. Review.
References
-
- E. Piovan, J. Yu, V. Tosello, D. Herranz, A. Ambesi-Impiombato, A. C. Da Silva, M. Sanchez-Martin, A. Perez-Garcia, I. Rigo, M. Castillo, S. Indraccolo, J. R. Cross, E. de Stanchina, E. Paietta, J. Racevskis, J. M. Rowe, M. S. Tallman, G. Basso, J. P. Meijerink, C. Cordon-Cardo, A. Califano, A. A. Ferrando,Direct reversal of glucocorticoid resistance by AKT inhibition in acute lymphoblastic leukemia. Cancer Cell 24,766–776 (2013). - PMC - PubMed
-
- O. R. Bandapalli, M. Zimmermann, C. Kox, M. Stanulla, M. Schrappe, W.-D. Ludwig, R. Koehler, M. U. Muckenthaler, A. E. Kulozik,NOTCH1 activation clinically antagonizes the unfavorable effect of PTEN inactivation in BFM-treated children with precursor T-cell acute lymphoblastic leukemia. Haematologica 98,928–936 (2013). - PMC - PubMed
-
- G. Wei, D. Twomey, J. Lamb, K. Schlis, J. Agarwal, R. W. Stam, J. T. Opferman, S. E. Sallan, M. L. den Boer, R. Pieters, T. R. Golub, S. A. Armstrong,Gene expression-based chemical genomics identifies rapamycin as a modulator of MCL1 and glucocorticoid resistance. Cancer Cell 10,331–342 (2006). - PubMed
-
- G. Tzoneva, A. Perez-Garcia, Z. Carpenter, H. Khiabanian, V. Tosello, M. Allegretta, E. Paietta, J. Racevskis, J. M. Rowe, M. S. Tallman, M. Paganin, G. Basso, J. Hof, R. Kirschner-Schwabe, T. Palomero, R. Rabadan, A. Ferrando,Activating mutations in the NT5C2 nucleotidase gene drive chemotherapy resistance in relapsed ALL. Nat. Med. 19,368–371 (2013). - PMC - PubMed
-
- J. A. Meyer, J. Wang, L. E. Hogan, J. J. Yang, S. Dandekar, J. P. Patel, Z. Tang, P. Zumbo, S. Li, J. Zavadil, R. L. Levine, T. Cardozo, S. P. Hunger, E. A. Raetz, W. E. Evans, D. J. Morrison, C. E. Mason, W. L. Carroll,Relapse-specific mutations in NT5C2 in childhood acute lymphoblastic leukemia. Nat. Genet. 45,290–294 (2013). - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

