SEC14-GOLD protein PATELLIN2 binds IRON-REGULATED TRANSPORTER1 linking root iron uptake to vitamin E
- PMID: 36493393
- PMCID: PMC10152663
- DOI: 10.1093/plphys/kiac563
SEC14-GOLD protein PATELLIN2 binds IRON-REGULATED TRANSPORTER1 linking root iron uptake to vitamin E
Abstract
Organisms require micronutrients, and Arabidopsis (Arabidopsis thaliana) IRON-REGULATED TRANSPORTER1 (IRT1) is essential for iron (Fe2+) acquisition into root cells. Uptake of reactive Fe2+ exposes cells to the risk of membrane lipid peroxidation. Surprisingly little is known about how this is avoided. IRT1 activity is controlled by an intracellular variable region (IRT1vr) that acts as a regulatory protein interaction platform. Here, we describe that IRT1vr interacted with peripheral plasma membrane SEC14-Golgi dynamics (SEC14-GOLD) protein PATELLIN2 (PATL2). SEC14 proteins bind lipophilic substrates and transport or present them at the membrane. To date, no direct roles have been attributed to SEC14 proteins in Fe import. PATL2 affected root Fe acquisition responses, interacted with ROS response proteins in roots, and alleviated root lipid peroxidation. PATL2 had high affinity in vitro for the major lipophilic antioxidant vitamin E compound α-tocopherol. Molecular dynamics simulations provided insight into energetic constraints and the orientation and stability of the PATL2-ligand interaction in atomic detail. Hence, this work highlights a compelling mechanism connecting vitamin E with root metal ion transport at the plasma membrane with the participation of an IRT1-interacting and α-tocopherol-binding SEC14 protein.
© The Author(s) 2022. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Conflict of interest statement
Conflict of interest statement. None declared.
Figures




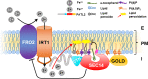
Comment in
-
Metal health: PATELLIN2 reduces iron-induced toxicity in Arabidopsis.Plant Physiol. 2023 May 2;192(1):15-16. doi: 10.1093/plphys/kiad090. Plant Physiol. 2023. PMID: 36789501 Free PMC article. No abstract available.
References
-
- Abraham MJ, Murtola T, Schulz R, Páll S, Smith JC, Hess B, Lindahl E (2015) GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1-2: 19–25. 10.1016/j.softx.2015.06.001 - DOI
-
- Atkinson J, Thakur V, Manor D (2019) The tocopherol transfer protein: regulator of vitamin E status. InWeber PBM, Blumberg J, Eggersdorfer M, Frank J, eds, Vitamin E in Human Health. Springer International Publishing, Cham, pp 111–124
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

