Immune cell interactions in tuberculosis
- PMID: 36493751
- PMCID: PMC12162144
- DOI: 10.1016/j.cell.2022.10.025
Immune cell interactions in tuberculosis
Abstract
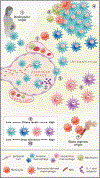
Despite having been identified as the organism that causes tuberculosis in 1882, Mycobacterium tuberculosis has managed to still evade our understanding of the protective immune response against it, defying the development of an effective vaccine. Technology and novel experimental models have revealed much new knowledge, particularly with respect to the heterogeneity of the bacillus and the host response. This review focuses on certain immunological elements that have recently yielded exciting data and highlights the importance of taking a holistic approach to understanding the interaction of M. tuberculosis with the many host cells that contribute to the development of protective immunity.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests Pending patent: A.K. Shalek, T. Hughes, M.H. Wadsworth, R. Seder, M. Roederer, J.L. Flynn, and P. Darrah, “COMPOSITIONS AND METHODS FOR TREATING BACTERIAL INFECTIONS,” US non-provisional patent application 17/137,481 claiming priority to US 62/954,998, filed December 30, 2020.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

