Molecular Biomarkers of Disease Outcomes and Mechanisms of Acquired Resistance to First-Line Osimertinib in Advanced EGFR-Mutant Lung Cancers
- PMID: 36494075
- PMCID: PMC10249779
- DOI: 10.1016/j.jtho.2022.11.022
Molecular Biomarkers of Disease Outcomes and Mechanisms of Acquired Resistance to First-Line Osimertinib in Advanced EGFR-Mutant Lung Cancers
Abstract
Introduction: Preferred first-line treatment for patients with metastatic EGFR-mutant lung cancer is osimertinib, yet it is not known whether patient outcomes may be improved by identifying and intervening on molecular markers associated with therapeutic resistance.
Methods: All patients with metastatic EGFR-mutant lung cancer treated with first-line osimertinib at the Memorial Sloan Kettering Cancer Center (n = 327) were identified. Available pretreatment and postprogression tumor samples underwent targeted gene panel sequencing and mutational signature analysis using SigMA algorithm. Progression-free survival (PFS) and overall survival were estimated using the Kaplan-Meier method.
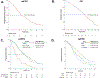
Results: Using multivariate analysis, baseline atypical EGFR (median PFS = 5.8 mo, p < 0.001) and concurrent TP53/RB1 alterations (median PFS = 10.5 mo, p = 0.015) were associated with shorter PFS on first-line osimertinib. Of 95 patients with postprogression biopsies, acquired resistance mechanisms were identified in 52% (off-target, n = 24; histologic transformation, n = 14; on-target, n = 12), with MET amplification (n = 9), small cell lung transformation (n = 7), and acquired EGFR amplification (n = 7), the most frequently identified mechanisms. Although there was no difference in postprogression survival on the basis of identified resistance (p = 0.07), patients with subsequent second-line therapy tailored to postprogression biopsy results had improved postprogression survival (hazard ratio = 0.09, p = 0.006). The paired postprogression tumors had higher tumor mutational burden (p = 0.008) and further dominant APOBEC mutational signatures (p = 0.07) compared with the pretreatment samples.
Conclusions: Patients with EGFR-mutant lung cancer treated with first-line osimertinib have improved survival with treatment adaptation on the basis of identified mechanisms of resistance at time of progression using tissue-based genomic analysis. Further survival gains may be achieved using risk-based treatment adaptation of pretreatment genomic alterations.
Keywords: EGFR; Mechanisms of resistance; Osimertinib; Targeted therapies; Tumor evolution.
Copyright © 2022 International Association for the Study of Lung Cancer. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
All other authors have declared no conflicts of interest.
Figures



Comment in
-
Resistance to EGFR Inhibitors: Fitness, Competition, and Diversity.J Thorac Oncol. 2023 Apr;18(4):390-392. doi: 10.1016/j.jtho.2023.01.006. J Thorac Oncol. 2023. PMID: 36990569 No abstract available.
References
-
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer Journal for Clinicians 2021;71:209–249. - PubMed
-
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA: A Cancer Journal for Clinicians 2022;72:7–33. - PubMed
-
- Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. New England Journal of Medicine 2019;382:41–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

