Biological properties of Staphylococcus virus ΦSA012 for phage therapy
- PMID: 36494564
- PMCID: PMC9734660
- DOI: 10.1038/s41598-022-25352-6
Biological properties of Staphylococcus virus ΦSA012 for phage therapy
Abstract
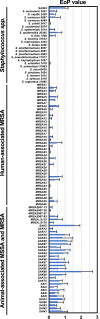
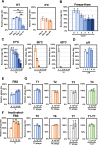
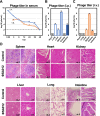
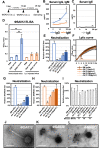
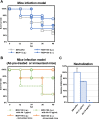
Staphylococcus virus ΦSA012 has a wide host range and efficient lytic activity. Here, we assessed the biological stability of ΦSA012 against temperature, freeze-thawing, and pH to clinically apply the phage. In addition, inoculation of ΦSA012 through i.p. and i.v. injections into mice revealed that phages were reached the limit of detection in serum and accumulated notably spleens without inflammation at 48 h post-inoculation. Furthermore, inoculation of ΦSA012 through s.c. injections in mice significantly induced IgG, which possesses neutralizing activity against ΦSA012 and other Staphylococcus viruses, ΦSA039 and ΦMR003, but not Pseudomonas viruses ΦS12-3 and ΦR18 or Escherichia viruses T1, T4, and T7 in vitro. Immunoelectron microscopic analysis showed that purified anti-phage IgG recognizes the long-tail fiber of staphylococcus viruses. Although S. aureus inoculation resulted in a 25% survival rate in a mouse i.p. model, ΦSA012 inoculation (i.p.) improved the survival rate to 75%; however, the survival rate of ΦSA012-immunized mice decreased to less than non-immunized mice with phage i.v. injection at a MOI of 100. These results indicated that ΦSA012 possesses promise for use against staphylococcal infections but we should carefully address the appropriate dose and periods of phage administration. Our findings facilitate understandings of staphylococcus viruses for phage therapy.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Isolation from sewage influent and characterization of novel Staphylococcus aureus bacteriophages with wide host ranges and potent lytic capabilities.Appl Environ Microbiol. 2009 Jul;75(13):4483-90. doi: 10.1128/AEM.02641-08. Epub 2009 May 1. Appl Environ Microbiol. 2009. PMID: 19411410 Free PMC article.
-
The Presence of Two Receptor-Binding Proteins Contributes to the Wide Host Range of Staphylococcal Twort-Like Phages.Appl Environ Microbiol. 2016 Sep 16;82(19):5763-74. doi: 10.1128/AEM.01385-16. Print 2016 Oct 1. Appl Environ Microbiol. 2016. PMID: 27422842 Free PMC article.
-
Bacteriophage ΦSA012 Has a Broad Host Range against Staphylococcus aureus and Effective Lytic Capacity in a Mouse Mastitis Model.Biology (Basel). 2018 Jan 9;7(1):8. doi: 10.3390/biology7010008. Biology (Basel). 2018. PMID: 29315249 Free PMC article.
-
Peculiarities of Staphylococcus aureus phages and their possible application in phage therapy.Appl Microbiol Biotechnol. 2019 Jun;103(11):4279-4289. doi: 10.1007/s00253-019-09810-2. Epub 2019 Apr 17. Appl Microbiol Biotechnol. 2019. PMID: 30997551 Review.
-
Exploring the therapeutic potential of staphylococcal phage formulations: Current challenges and applications in phage therapy.J Appl Microbiol. 2022 May;132(5):3515-3532. doi: 10.1111/jam.15462. Epub 2022 Feb 6. J Appl Microbiol. 2022. PMID: 35064991 Review.
Cited by
-
A New Approach for Phage Cocktail Design in the Example of Anti-Mastitis Solution.Pathogens. 2024 Sep 27;13(10):839. doi: 10.3390/pathogens13100839. Pathogens. 2024. PMID: 39452711 Free PMC article.
-
Protocol for end-design-free rebooting of terminally redundant Pseudomonas phages in clinical isolates of Pseudomonas aeruginosa.STAR Protoc. 2025 Aug 4;6(3):104012. doi: 10.1016/j.xpro.2025.104012. Online ahead of print. STAR Protoc. 2025. PMID: 40763034 Free PMC article.
-
Precise microbiome engineering using natural and synthetic bacteriophages targeting an artificial bacterial consortium.Front Microbiol. 2024 May 2;15:1403903. doi: 10.3389/fmicb.2024.1403903. eCollection 2024. Front Microbiol. 2024. PMID: 38756723 Free PMC article.
-
Phage therapy: a promising approach for Staphylococcus aureus diabetic foot infections.J Virol. 2025 Jun 17;99(6):e0045825. doi: 10.1128/jvi.00458-25. Epub 2025 May 14. J Virol. 2025. PMID: 40366171 Free PMC article. Review.
-
Phage-specific antibodies: are they a hurdle for the success of phage therapy?Essays Biochem. 2024 Dec 17;68(5):633-644. doi: 10.1042/EBC20240024. Essays Biochem. 2024. PMID: 39254211 Free PMC article. Review.
References
-
- Yamane I. Epidemiological survey and economical evaluation of bo- vine mastitis in tie-stall dairy farms. J. Jpn. Vet. Med. Assoc. 2006;59:674–678. doi: 10.12935/jvma1951.59.674. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

