Two-year follow-up of KTE-X19 in patients with relapsed or refractory adult B-cell acute lymphoblastic leukemia in ZUMA-3 and its contextualization with SCHOLAR-3, an external historical control study
- PMID: 36494725
- PMCID: PMC9734710
- DOI: 10.1186/s13045-022-01379-0
Two-year follow-up of KTE-X19 in patients with relapsed or refractory adult B-cell acute lymphoblastic leukemia in ZUMA-3 and its contextualization with SCHOLAR-3, an external historical control study
Abstract
Background: Brexucabtagene autoleucel (KTE-X19) is an autologous anti-CD19 CAR T-cell therapy approved in the USA to treat adult patients with relapsed or refractory B-precursor acute lymphoblastic leukemia (R/R B-ALL) based on ZUMA-3 study results. We report updated ZUMA-3 outcomes with longer follow-up and an extended data set along with contextualization of outcomes to historical standard of care.
Methods: Adults with R/R B-ALL received a single infusion of KTE-X19 (1 × 106 CAR T cells/kg). Long-term post hoc subgroup assessments of ZUMA-3 were conducted. Outcomes from matched patients between historical clinical trials and ZUMA-3 patients were assessed in the retrospective historical control study SCHOLAR-3.
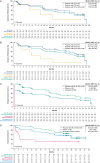
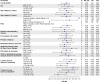
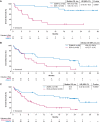
Results: After 26.8-months median follow-up, the overall complete remission (CR) rate (CR + CR with incomplete hematological recovery) among treated patients (N = 55) in phase 2 was 71% (56% CR rate); medians for duration of remission and overall survival (OS) were 14.6 and 25.4 months, respectively. Most patients responded to KTE-X19 regardless of age or baseline bone marrow blast percentage, but less so in patients with > 75% blasts. No new safety signals were observed. Similar outcomes were observed in a pooled analysis of phase 1 and 2 patients (N = 78). In SCHOLAR-3, the median OS for treated patients from ZUMA-3 (N = 49) and matched historical controls (N = 40) was 25.4 and 5.5 months, respectively.
Conclusions: These data, representing the longest follow-up of CAR T-cell therapy in a multicenter study of adult R/R B-ALL, suggest that KTE-X19 provides a clinically meaningful survival benefit with manageable toxicity in this population.
Trial registration: NCT02614066.
Keywords: B-precursor acute lymphoblastic leukemia; Brexucabtagene autoleucel; CAR T-cell therapy; KTE-X19; SCHOLAR-3; ZUMA-3.
© 2022. The Author(s).
Conflict of interest statement
BDS reports honoraria from Acrotech, Beigene, Gilead Sciences, Janssen, Pharmacyclics, and Spectrum; consulting/advisory role for Adaptive Biotechnologies, Amgen, Bristol-Myers Squibb (BMS)/Celgene, Kite, Novartis, Pfizer, and Precision Biosciences; research funding from Gilead Sciences, Incyte, Jazz Pharmaceuticals, and Kite; and travel support from Celgene, Janssen, Kite, Novartis, Pfizer, Seattle Genetics, and Stemline Therapeutics. AG reports consulting/advisory role for Amgen, Atara, Celgene, Kite, and Wugen Inc.; research funding from Amgen and Kite; and honoraria from Kite. OOO reports research funding from Kite and consulting/advisory role for Janssen, Pfizer, Novartis, Curio science, ADC therapeutics, TG therapeutics. ACL reports consulting/advisory role for Gilead, Kite, Roche, BMS/Celgene, Incyte, Takeda; research funding from Roche and Celgene; and travel support from Roche and Kite. NB reports honoraria and consulting or advisory role for Gilead. RDC reports employment with Seagen; stock or other ownership in Seagen; honoraria from Amgen, Kite, and Pfizer; consulting/advisory role for Amgen; research funding from Pfizer, Amgen, Kite, Merck, Pfizer, Servier, and Vanda. TL reports consultancy/advisory role for Amgen and Servier. MiRB reports honoraria from Agios, BMS, Celgene, Kite, Incyte, Novartis, and Sanofi; consulting/advisory role for Agios, Arcellx, Bluebird Bio, CRISPR Therapeutics, Iovance, Kite, Novartis, and WindMIL Therapeutics; speakers' bureau participation for Agios, BMS, Incyte, Kite, and Sanofi; research funding from Arcellx, Autolus, CRISPR Therapeutics, Immatics, Kite, Novartis, Triumvira, and Tmunity; and travel support from Agios, BMS, Incyte, Kite, and Novartis. MST reports consulting/advisory role for Amgen, Celgene, Kite, Regeneron, and Roche and research funding from Amgen, Kite, MacroGenics, Regeneron, and Roche. DT reports consulting/advisory role for BMS, EUSA, Partner, and Takeda, and research funding from BMS. KMO reports consulting/advisory role for BEAM Therapeutics. MLA reports consulting/advisory role for Kite and Syndax Pharmaceuticals, Inc.; research funding from Kite (Institutional PI). YL reports consulting/advisory role for Bluebird Bio, Celgene, Gamida Cell, Janssen, Novartis, Juno, Kite, Legend, Sorrento, and Vineti; research funding from Bluebird Bio, Celgene, Janssen, Kite, Merck, and Takeda. MaRB reports research funding from Abbvie, Ascentage, Kite, Kura, and Takeda. GJS reports stock or other ownership in Amgen, BMS, and Johnson & Johnson; honoraria from Agios, Amgen, AstraZeneca, Novartis, and Ono Pharma; consulting/advisory role for Agios, Celgene, and Jazz; speakers' bureau participation for Astellas, AbbVie, BMS, Celgene, Incyte, Karyopharm, Kite, and Stemline; research funding from AbbVie, Actinium, Actuate, Arog, Astellas, AVM Biopharma, Celgene, Cellectis, Cellerant, Constellation, CTI, Cyclacel, Forma, Daiichi-Sankyo, Deciphera, Delta-fly, FujiFilm, Gamida, Genentech/Roche, Geron, Gilead, Glycomimetics, Incyte, Janssen, Karyopharm, Kite, Mateon, Medimmune, Millennium, Novartis, Onconova, Pfizer, PreCOG, Regimmune, Samus, Sangamo, Sellas, Stemline, Takeda, Tolero, and Trovagene. JHP reports consulting/advisory role for AstraZeneca, Kite, and Novartis, and research funding from Amgen, Genentech, and Juno. MS reports consulting/advisory role for Amgen, Celgene/BMS, Gilead, Janssen, and Novartis; speakers' bureau participation for BMS/Celgene, Gilead, Janssen, and Novartis; research funding from Amgen, Gilead, Miltenyi Biotech, Morphosys, Roche, and Seattle Genetics; and travel support from Takeda. MA reports stock or other ownership with Cytodyn; consulting/advisory role for Celgene; speakers' bureau participation for Celgene, BMS, Abbvie, and Kite. MCM reports consulting/advisory role for BMS, and Janssen-Cilag; speakers bureau participation for Janssen-Cilag, and Medscape; WGW reports consulting/advisory role for Genzyme and Sanofi, and research funding from AbbVie, Acerta, Cyclacel, Genentech, Gilead, GlaxoSmithKline, Janssen, Juno, Karyopharm, Loxo Oncology, miRagen, Novartis, Oncternal, Pharmacyclics, Sunesis, and Xencor. DJD reports consulting or advisory role for Agios, Amgen, Autolos, Blueprint, Forty-Seven, Gilead, Incyte, Jazz, Novartis, Pfizer, Shire, and Takeda; research funding from AbbVie, Glycomimetics, Novartis and Blueprint Pharmaceuticals. PS reports honoraria from Karyopharm and MorphoSys; consulting/advisory role for CRISPR Therapeutics, Karyopharm, and MorphoSys; and research funding from Amgen, BMS, Gamida-Cell, Kite, Janssen, Macrogenics, and Seattle Genetics. DJ reports research funding from Jazz Pharmaceuticals and Pfizer. JD reports employment with Kite; stock or other ownership in Gilead Sciences; consulting/advisory role for GliaCure; and patents, royalties, or other intellectual property from: Patent US8598141 (Dec 03, 2013). SA reports employment and stock or other ownership with Kite. LZ reports employment with Kite and stock or other ownership in and travel support from AbbVie. PS reports employment with Kite; stock or other ownership in Gilead Sciences; and travel support from Kite and Gilead Sciences. IF reports employment with Kite; stock or other ownership of Gilead and Roche. BKM reports employment with and travel support from Kite, and stock or other ownership in GlaxoSmithKline, Kite, and Lava. RH reports honoraria from ADC therapeutics, BMS, Celgene, Gilead, Janssen, Kite, MSD, Novartis, and Roche, and consulting/advisory role for Kite/Gilead.
Figures
References
-
- Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab ozogamicin versus standard of care in relapsed or refractory acute lymphoblastic leukemia: final report and long-term survival follow-up from the randomized, phase 3 INO-VATE study. Cancer. 2019;125(14):2474–2487. doi: 10.1002/cncr.32116. - DOI - PMC - PubMed
-
- Acute Lymphocytic Leukemia (ALL) SEER 5-Year Relative Survival Rates, 2012–2018. seer.cancer.gov/statistics. Accessed November 2022.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

