IMAGENE trial: multicenter, proof-of-concept, phase II study evaluating the efficacy and safety of combination therapy of niraparib with PD-1 inhibitor in solid cancer patients with homologous recombination repair genes mutation
- PMID: 36494792
- PMCID: PMC9733213
- DOI: 10.1186/s12885-022-10398-6
IMAGENE trial: multicenter, proof-of-concept, phase II study evaluating the efficacy and safety of combination therapy of niraparib with PD-1 inhibitor in solid cancer patients with homologous recombination repair genes mutation
Abstract
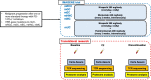
Background: Previous clinical trials have demonstrated the potential efficacy of poly (ADP-ribose) polymerase (PARP) inhibitors (PARPis) in patients with cancer involving homologous recombination repair (HRR) gene-mutation. Moreover, HRR gene-mutated cancers are effectively treated with immune checkpoint inhibitors (ICIs) with the increase in tumor mutation burden. We have proposed to conduct a multicenter, single-arm phase II trial (IMAGENE trial) for evaluating the efficacy and safety of niraparib (PARPi) plus programmed cell death-1 inhibitor combination therapy in patients with HRR gene-mutated cancers who are refractory to ICIs therapy using a next generation sequencing-based circulating tumor DNA (ctDNA) and tumor tissue analysis.
Methods: Key eligibility criteria for this trial includes HRR gene-mutated tumor determined by any cancer gene tests; progression after previous ICI treatment; and Eastern Cooperative Oncology Group Performance Status ≤ 1. The primary endpoint is the confirmed objective response rate (ORR) in all patients. The secondary endpoints include the confirmed ORR in patients with HRR gene-mutation of ctDNA using the Caris Assure (CARIS, USA). The target sample size of the IMAGENE trial is 57 patients. Biomarker analyses will be performed in parallel using the Caris Assure, proteome analysis, and T cell repertoire analysis to reveal tumor immunosurveillance in peripheral blood.
Expected outcome: Our trial aims to confirm the clinical benefit of PARPi plus ICI combination therapy in ICI-resistant patients. Furthermore, through translational research, our trial will shed light on which patients would benefit from the targeted combination therapy for patients with HRR gene-mutated tumor even after the failure of ICIs.
Trial registration: The IMAGENE trial: jRCT, Clinical trial no.: jRCT2051210120, Registered date: November 9, 2021.
Keywords: Homologous recombination repair; Immune checkpoint inhibitor; Niraparib; PARP inhibitor; Tumor-agnostic therapy.
© 2022. The Author(s).
Conflict of interest statement
TK reports research funding supports from Takeda and Pfizer. NM reports honoraria from Janssen, Merck biopharma, and Sanofi and research funding supports from Janssen, AstraZeneca, Bayer, Roche, MSD, Taiho, Astellas, Amgen, Eisai, Eli Lilly, PRA Health Science, Takeda, Pfizer, Seagen, Chugai, Abbvie, and Novartis. MS reports honoraria from Janssen, AstraZeneca, and Astellas and research funding support from Daiichi Sankyo. ME reports honoraria from ONO, Takeda, Novartis, Pfizer, Bristol, Janssen, MSD, Merck, AstraZeneca, and Eisai and research funding support from Sanofi, Bayer, Astellas, ONO, and Takeda. TO reports honoraria from Takeda. MO reports honoraria from Bayer, Bristol, Novartis, Ono, Merck, Takeda, MSD, and Pfizer. KN reports honoraria from Ono, Novartis, MSD, and Bristol, and consulting fees from Novartis and MSD. YN reports honoraria from Chugai, Merck Biopharma, and Guardant Health AMEA, and research funding support from Taiho, Chugai, Guardant Health, Genomedia, Daiichi Sankyo, Seagen, and Roche Diagnostics. HB reports honoraria from Taiho, Ono, and Eli Lilly Japan, and research funding support from Ono. TY reports honoraria from Chugai, Merck, Bayer, Ono, and MSD, and research funding from MSD, Daiichi Sankyo, Ono, Taiho, Amgen, Sanofi, Pfizer, Genomedia, Sysmex, Chugai, and Nippon Boehringer Ingelheim. NN reports honoraria from Takeda, Janssen, AstraZeneca, Merck Biopharma, Ono, and Bristol.
Figures
References
-
- Murai J, Pommier Y. PARP trapping beyond homologous recombination and platinum sensitivity in cancers. Ann Rev Cancer Biol. 2019;3:131–150. doi: 10.1146/annurev-cancerbio-030518-055914. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

