Impact of chemotherapy and/or immunotherapy on neutralizing antibody response to SARS-CoV-2 mRNA-1237 vaccine in patients with solid tumors
- PMID: 36495129
- PMCID: PMC9877816
- DOI: 10.1002/1878-0261.13359
Impact of chemotherapy and/or immunotherapy on neutralizing antibody response to SARS-CoV-2 mRNA-1237 vaccine in patients with solid tumors
Abstract
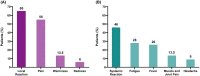
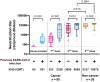
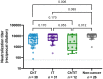
Patients with solid tumors have been a risk group since the beginning of the SARS-CoV-2 pandemic due to more significant complications, hospitalizations or deaths. The immunosuppressive state of cancer treatments or the tumor itself could influence the development of post-vaccination antibodies. This study prospectively analyzed 89 patients under chemotherapy and/or immunotherapy, who received two doses of the mRNA-1237 vaccine, and were compared with a group of 26 non-cancer individuals. Information on adverse events and neutralizing antibodies against the ancestral strain of SARS-CoV-2 (WH1) have been analyzed. Local reactions accounted for 65%, while systemic reactions accounted for 46% of oncologic individuals/cancer patients. Regarding the response to vaccination, 6.7% of cancer patients developed low neutralizing antibody levels. Lower levels of neutralizing antibodies between cancer and non-cancer groups were significant in individuals without previous SARS-CoV-2 infection, but not in previously infected individuals. We also observed that patients receiving chemotherapy or chemoimmunotherapy have significantly lower levels of neutralizing antibodies than non-cancer individuals. In conclusion, our study confirms the importance of prioritizing cancer patients receiving anticancer treatment in SARS-CoV-2 vaccination programs.
Keywords: COVID-19; adverse reactions; anticancer therapy; cancer; moderna vaccine; neutralizing antibodies.
© 2022 The Authors. Molecular Oncology published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
Outside the submitted work JB is founder and shareholder of AlbaJuna Therapeutics, S.L. BC is founder and shareholder of AlbaJuna Therapeutics, S.L and AELIX Therapeutics, S.L. EF declares advisory role fees from Novartis; travel expenses fees from Lilly, Novartis and Pfizer; and a research funding from Pfizer. MB declares consulting/advisory role fees from Roche, Bristol‐Myers, Boehringer, AstraZeneca, Lilly, and a research funding from Kyowa Kirin. The other authors declare no conflict of interest.
Figures
Similar articles
-
mRNA-1273 COVID-19 vaccination in patients receiving chemotherapy, immunotherapy, or chemoimmunotherapy for solid tumours: a prospective, multicentre, non-inferiority trial.Lancet Oncol. 2021 Dec;22(12):1681-1691. doi: 10.1016/S1470-2045(21)00574-X. Epub 2021 Nov 9. Lancet Oncol. 2021. PMID: 34767759 Free PMC article. Clinical Trial.
-
Decreased and Heterogeneous Neutralizing Antibody Responses Against RBD of SARS-CoV-2 Variants After mRNA Vaccination.Front Immunol. 2022 Apr 6;13:816389. doi: 10.3389/fimmu.2022.816389. eCollection 2022. Front Immunol. 2022. PMID: 35464418 Free PMC article.
-
Hybrid Immunity Shifts the Fc-Effector Quality of SARS-CoV-2 mRNA Vaccine-Induced Immunity.mBio. 2022 Oct 26;13(5):e0164722. doi: 10.1128/mbio.01647-22. Epub 2022 Aug 24. mBio. 2022. PMID: 36000735 Free PMC article.
-
Brief Research Report: Anti-SARS-CoV-2 Immunity in Long Lasting Responders to Cancer Immunotherapy Through mRNA-Based COVID-19 Vaccination.Front Immunol. 2022 Jul 5;13:908108. doi: 10.3389/fimmu.2022.908108. eCollection 2022. Front Immunol. 2022. PMID: 35911701 Free PMC article.
-
Synergistic Action of Immunotherapy and Nanotherapy against Cancer Patients Infected with SARS-CoV-2 and the Use of Artificial Intelligence.Cancers (Basel). 2022 Jan 2;14(1):213. doi: 10.3390/cancers14010213. Cancers (Basel). 2022. PMID: 35008377 Free PMC article. Review.
Cited by
-
Neutralizing antibodies and safety of a COVID-19 vaccine against SARS-CoV-2 wild-type and Omicron variants in solid cancer patients.PLoS One. 2024 Nov 7;19(11):e0310781. doi: 10.1371/journal.pone.0310781. eCollection 2024. PLoS One. 2024. PMID: 39509358 Free PMC article.
-
Neutralizing Antibody Response following a Third Dose of the mRNA-1273 Vaccine among Cancer Patients.Vaccines (Basel). 2023 Dec 22;12(1):13. doi: 10.3390/vaccines12010013. Vaccines (Basel). 2023. PMID: 38250826 Free PMC article.
-
Global seroprevalence of neutralizing antibodies against adeno-associated virus serotypes used for human gene therapies.Mol Ther Methods Clin Dev. 2024 May 29;32(3):101273. doi: 10.1016/j.omtm.2024.101273. eCollection 2024 Sep 12. Mol Ther Methods Clin Dev. 2024. PMID: 39022744 Free PMC article.
References
-
- Khoury E, Nevitt S, Madsen WR, Turtle L, Davies G, Palmieri C. Differences in outcomes and factors associated with mortality among patients with SARS‐CoV‐2 infection and cancer compared with those without cancer: a systematic review and meta‐analysis. JAMA Netw Open. 2022;5(5):e2210880. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

