Impact of Genetic Diagnosis on the Outcome of Hematopoietic Stem Cell Transplant in Primary Immunodeficiency Disorders
- PMID: 36495401
- PMCID: PMC9958161
- DOI: 10.1007/s10875-022-01403-5
Impact of Genetic Diagnosis on the Outcome of Hematopoietic Stem Cell Transplant in Primary Immunodeficiency Disorders
Abstract
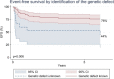
To evaluate the relationship between knowledge of genetic diagnosis before HSCT and outcome, we reviewed all HSCTs for primary immune deficiencies (PID) performed at UCSF from 2007 through 2018. SCID, a distinct entity identified since 2010 in California by newborn screening and treated early, was considered separately. The underlying genetic condition was known at the time of HSCT in 85% of cases. Graft failure was less frequent in patients with a genetic diagnosis (19% with a genetic diagnosis versus 47% without, p = 0.020). Furthermore, event-free survival and overall survival (OS) at 5 years were better for those with a genetic diagnosis (78% with versus 44% without, p = 0.006; and 93% versus 60% without, p = 0.0002, respectively). OS at 5 years was superior for known-genotype patients with both SCID (p = 0.010) and non-SCID PID (p = 0.010). There was no difference in OS between HSCT done in 2007-2010 compared to more recently (p = 0.19). These data suggest that outcomes of HSCT for PID with known genotype may reflect specific experience and literature, or that a substantial proportion of patients with PID of undetermined genotype may have had underlying conditions for which HSCT may carry greater risk. The higher rate of graft failure in PID with unknown genotype may be in part explained by insufficient conditioning, which in turn could be dictated by compromised organ function in patients undergoing HSCT late in the course. Widespread availability of PID gene sequencing as standard care can provide genetic diagnoses for most patients with PID prior to HSCT, permitting optimization of transplant approach.
Keywords: Primary immunodeficiency; genotype; outcome; transplant.
© 2022. The Author(s).
Conflict of interest statement
FF and JD have no conflict of interest. MJD has consulted for Horizon Therapeutics, and Chiesi Inc.t.; CCD has consulted for Alexion Inc. and Omeros Corporation and Jazz Pharmaceuticals; JMP is a writer and editor for UpToDate, and her spouse is employed by and owns stock in Invitae. MJC is a writer for UpToDate and is on advisory boards for bluebird bio, Homology Medicine Inc (owns stock), Chiesi Inc, and Rocket Pharma. AC has consulted for Invitae and Thymmune and Enzyvant.
Figures



References
-
- Tangye SG, Al-Herz W, Bousfiha A, Chatila T, Cunningham-Rundles C, Etzioni A, et al. Human inborn errors of immunity: 2019 update on the classification from the International Union of Immunological Societies Expert Committee. J Clin Immunol. 2020;40(1):24–64. doi: 10.1007/s10875-019-00737-x. - DOI - PMC - PubMed
-
- Chan AY, Leiding JW, Liu X, Logan BR, Burroughs LM, Allenspach EJ, et al. Hematopoietic cell transplantation in patients with primary immune regulatory disorders (PIRD): a primary immune deficiency treatment consortium (PIDTC) Survey. Front Immunol. 2020;21(11):239–239. doi: 10.3389/fimmu.2020.00239. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

