The effects of preconception and early gestation SARS-CoV-2 infection on pregnancy outcomes and placental pathology
- PMID: 36495735
- PMCID: PMC9721196
- DOI: 10.1016/j.anndiagpath.2022.152076
The effects of preconception and early gestation SARS-CoV-2 infection on pregnancy outcomes and placental pathology
Abstract
Objective: To evaluate if peri-pregnancy timing of a PCR+ test for SARS-CoV-2 RNA affects pregnancy outcomes and placental pathology.
Methods: This is a retrospective cohort study conducted in a tertiary center. Pregnancy outcomes and placental pathology were compiled for women who tested positive for SARS-CoV-2 RNA from a nasopharyngeal swab assessed by RT-PCR. The population comprised four groups that were PCR+ preconception (T0) or in the 1st (T1), 2nd (T2), or 3rd (T3) trimester of pregnancy. A fifth, control group (TC) tested PCR- for SARS-CoV-2 before delivery.
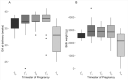
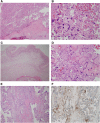
Results: Seventy-one pregnancies were studied. The T0 group exhibited lower gestational ages at delivery, had infants with the lowest birth weights, the highest rate of pregnancy loss before 20 weeks. Features of maternal vascular malperfusion and accelerated villous maturation were prominent findings in the histopathology of placentas from women PCR+ for SARS-CoV-2 RNA, especially in the T0 and the T1 groups.
Conclusion: Women at highest risk for pregnancy complications are those who test PCR+ for viral RNA preconception or during first trimester of pregnancy.
Keywords: COVID-19; Neonatal outcome; Placental pathology; Pregnancy outcome; SARS-CoV-2; Timing.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest All co-authors declare no conflict of interest. This study was supported by faculty developmental fund to Dr. Mai He by the Department of Pathology & Immunology, Washington University in St. Louis School of Medicine.
Figures
Similar articles
-
Analysis of placental pathology after COVID-19 by timing and severity of infection.Am J Obstet Gynecol MFM. 2023 Jul;5(7):100981. doi: 10.1016/j.ajogmf.2023.100981. Epub 2023 Apr 23. Am J Obstet Gynecol MFM. 2023. PMID: 37094637 Free PMC article.
-
Association Between COVID-19 Pregnant Women Symptoms Severity and Placental Morphologic Features.Front Immunol. 2021 May 26;12:685919. doi: 10.3389/fimmu.2021.685919. eCollection 2021. Front Immunol. 2021. PMID: 34122449 Free PMC article.
-
SARS-CoV-2 and Placental Pathology: Malperfusion Patterns Are Dependent on Timing of Infection During Pregnancy.Am J Surg Pathol. 2022 Jan 1;46(1):51-57. doi: 10.1097/PAS.0000000000001772. Am J Surg Pathol. 2022. PMID: 34310367 Free PMC article.
-
SARS-CoV-2 Infection and Placental Pathology.Rev Bras Ginecol Obstet. 2021 Jun;43(6):474-479. doi: 10.1055/s-0041-1730291. Epub 2021 Jun 2. Rev Bras Ginecol Obstet. 2021. PMID: 34077991 Free PMC article. Review.
-
Placental Vascular and Inflammatory Findings from Pregnancies Diagnosed with Coronavirus Disease 2019: A Systematic Review and Meta-analysis.Am J Perinatol. 2022 Nov;39(15):1643-1653. doi: 10.1055/a-1787-7933. Epub 2022 Mar 3. Am J Perinatol. 2022. PMID: 35240710
Cited by
-
Host factors of SARS-CoV-2 in infection, pathogenesis, and long-term effects.Front Cell Infect Microbiol. 2024 May 22;14:1407261. doi: 10.3389/fcimb.2024.1407261. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38846354 Free PMC article. Review.
-
COVID-19 in pregnant women: a systematic review and meta-analysis on the risk and prevalence of pregnancy loss.Hum Reprod Update. 2024 Mar 1;30(2):133-152. doi: 10.1093/humupd/dmad030. Hum Reprod Update. 2024. PMID: 38016805 Free PMC article.
References
-
- Khong T.Y., Mooney E.E., Ariel I., et al. Sampling and definitions of placental lesions: Amsterdam placental workshop group consensus statement. Arch Pathol Lab Med. 2016;140:698–713. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

