Differential dose responses of transcranial focused ultrasound at brain regions indicate causal interactions
- PMID: 36496128
- PMCID: PMC9806861
- DOI: 10.1016/j.brs.2022.12.003
Differential dose responses of transcranial focused ultrasound at brain regions indicate causal interactions
Abstract
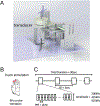
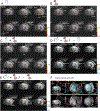
We have previously shown that focused ultrasound (FUS) pulses in low pressure range exerted bidirectional and brain state-dependent neuromodulation in the nonhuman primate somatosensory cortices by fMRI. Here we aim to gain insights about the proposed neuron selective modulation of FUS and probe feedforward versus feedback interactions by simultaneously quantifying the stimulus (FUS pressures: 925, 425, 250 kPa) and response (% BOLD fMRI changes) function at the targeted area 3a/3b and off-target cortical areas at 7T. In resting-state, lowered intensities of FUS resulted in decreased fMRI signal changes at the target area 3a/3b and off-target area 1/2, S2, MCC, insula and auditory cortex, and no signal difference in thalamic VPL and MD nuclei. In activated states, concurrent high-intensity FUS significantly enhanced touch-evoked signals in area 1/2. Medium- and low-intensity FUS significantly suppressed touch-evoked BOLD signals in all areas except in the auditory cortex, VPL and MD thalamic nuclei. Distinct state dependent and dose-response curves led us to hypothesize that FUS's neuromodulatory effects may be mediated through preferential activation of different populations of neurons. Area 3a/3b may have distinct causal feedforward and feedback interactions with Area 1/2, S2, MCC, insula, and VPL. FUS offers a noninvasive neural stimulation tool for dissecting brain circuits and probing causal functional connections.
Keywords: Brain circuit; Hand; Neuromodulation; Primates; Somatosensory; Transcranial focused ultrasound; fMRI.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare no competing interests.
Figures
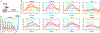
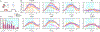


Similar articles
-
Disrupting nociceptive information processing flow through transcranial focused ultrasound neuromodulation of thalamic nuclei.Brain Stimul. 2023 Sep-Oct;16(5):1430-1444. doi: 10.1016/j.brs.2023.09.013. Epub 2023 Sep 21. Brain Stimul. 2023. PMID: 37741439 Free PMC article.
-
Bidirectional and state-dependent modulation of brain activity by transcranial focused ultrasound in non-human primates.Brain Stimul. 2021 Mar-Apr;14(2):261-272. doi: 10.1016/j.brs.2021.01.006. Epub 2021 Jan 15. Brain Stimul. 2021. PMID: 33460838 Free PMC article.
-
Neuromodulation of sensory networks in monkey brain by focused ultrasound with MRI guidance and detection.Sci Rep. 2018 May 22;8(1):7993. doi: 10.1038/s41598-018-26287-7. Sci Rep. 2018. PMID: 29789605 Free PMC article.
-
Percept-related activity in the human somatosensory system: functional magnetic resonance imaging studies.Magn Reson Imaging. 2004 Dec;22(10):1539-48. doi: 10.1016/j.mri.2004.10.003. Magn Reson Imaging. 2004. PMID: 15707803 Review.
-
Neuromodulation with transcranial focused ultrasound.Neurosurg Focus. 2018 Feb;44(2):E14. doi: 10.3171/2017.11.FOCUS17621. Neurosurg Focus. 2018. PMID: 29385924 Free PMC article. Review.
Cited by
-
Functional ultrasound (fUS) imaging of displacement-guided focused ultrasound (FUS) neuromodulation in mice.bioRxiv [Preprint]. 2024 Apr 1:2024.03.29.587355. doi: 10.1101/2024.03.29.587355. bioRxiv. 2024. Update in: Neuroimage. 2024 Sep;298:120768. doi: 10.1016/j.neuroimage.2024.120768. PMID: 38617295 Free PMC article. Updated. Preprint.
-
Low intensity transcranial ultrasound stimulation induces hemodynamic responses through neurovascular coupling.iScience. 2024 Jun 17;27(7):110269. doi: 10.1016/j.isci.2024.110269. eCollection 2024 Jul 19. iScience. 2024. PMID: 39055926 Free PMC article.
-
Disrupting nociceptive information processing flow through transcranial focused ultrasound neuromodulation of thalamic nuclei.Brain Stimul. 2023 Sep-Oct;16(5):1430-1444. doi: 10.1016/j.brs.2023.09.013. Epub 2023 Sep 21. Brain Stimul. 2023. PMID: 37741439 Free PMC article.
-
Generating Patient-Specific Acoustic Simulations for Transcranial Focused Ultrasound Procedures Based on Optical Tracking Information.IEEE Open J Ultrason Ferroelectr Freq Control. 2023;3:146-156. doi: 10.1109/ojuffc.2023.3318560. Epub 2023 Sep 25. IEEE Open J Ultrason Ferroelectr Freq Control. 2023. PMID: 38222464 Free PMC article.
-
The relationship between parameters and effects in transcranial ultrasonic stimulation.ArXiv [Preprint]. 2024 Jul 3:arXiv:2407.01232v2. ArXiv. 2024. Update in: Brain Stimul. 2024 Nov-Dec;17(6):1216-1228. doi: 10.1016/j.brs.2024.10.008. PMID: 39010874 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

