Characterization of immunologically detectable T-cell sensitization, Immunohistochemical detection of pro-inflammatory cytokines, and clinical parameters of patients after allogeneic intraoral bone grafting procedures: a prospective randomized controlled clinical trial in humans
- PMID: 36496367
- PMCID: PMC9741780
- DOI: 10.1186/s12903-022-02584-6
Characterization of immunologically detectable T-cell sensitization, Immunohistochemical detection of pro-inflammatory cytokines, and clinical parameters of patients after allogeneic intraoral bone grafting procedures: a prospective randomized controlled clinical trial in humans
Abstract
Background: The null hypotheses were tested that intraoral bone augmentation using two different allogeneic materials has no impact on the patient's blood levels of material-specific lymphocytes and on the immunohistochemical detection of pro-inflammatory cytokines IL-1α, IL1ß and TNF-α and T-cell markers CD4, CD8 in biopsies of the test groups.
Methods: In this prospective RCT, 60 systemically healthy participants were randomly assigned to two allogeneic test groups (1: Maxgraft®, freeze-dried, multiple donors, and 2: Puros®, solvent-dehydrated, single donor) and an autologous control group (10 patients). Plasma samples were collected pre-(T1) and postoperatively (2 weeks (T2) and 4 months (T3)). The Lymphocyte Transformation Test (LTT) was used for analyzing levels of transformed lymphocytes for type IV immune reactions by 3H-thymidine activity. Bone biopsies were harvested at T3 and immunohistochemically analyzed for IL-1α, IL1ß, TNF-α, CD4, CD8 and correlated with the immunological and clinical findings.
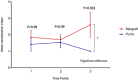
Results: A statistically significant difference between the tested materials was observed for LTT measurements at T3 (p = 0.033). Furthermore, three groups were identified: Group A (LTT negative T1-T3, n = 48), group B (LTT positive T1-T3, n = 7), group C (developing positive LTT at T2, n = 5). A highly significant elevation of IL-1α, IL1ß, TNF-α in patients of group C (p = 0.0001) and a significant elevation of CD4+ cells in patients of group B (p = 0.005) was shown.
Conclusion: Our data show that following allogeneic bone grafting, local and systemic immunological reactions can be detected in some patients. These findings were statistically significant for the timepoint T3 between the tested materials as well as for the groups B and C correlated with group A for both tested materials. Therefore, the null hypotheses were rejected. A preoperative compatibility test for allogeneic materials in order to improve patient safety and the predictability of these materials would be desirable.
Trial registration: Ethical commission of the Ärztekammer Hamburg, Germany (PV5211) as well as by the German Registry of Clinical Studies (DRKS00013010) on 30/07/2018 ( http://apps.who.int/trialsearch/ ).
Keywords: Allogeneic bone graft; Alloimmunization; Human histology; Immunohistochemistry; Lymphocyte transformation test.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests and no conflict of interest.
Figures


Similar articles
-
Characterization of circulating molecules and activities in plasma of patients after allogeneic and autologous intraoral bone grafting procedures: a prospective randomized controlled clinical trial in humans.BMC Oral Health. 2022 Jan 30;22(1):24. doi: 10.1186/s12903-021-02036-7. BMC Oral Health. 2022. PMID: 35094679 Free PMC article. Clinical Trial.
-
Impact of allogenic and autologous transfusion on immune function in patients with tumors.Asian Pac J Cancer Prev. 2014;15(1):467-74. doi: 10.7314/apjcp.2014.15.1.467. Asian Pac J Cancer Prev. 2014. PMID: 24528076
-
Histological and immunohistochemical comparison of two different allogeneic bone grafting materials for alveolar ridge reconstruction: A prospective randomized trial in humans.Clin Implant Dent Relat Res. 2019 Oct;21(5):1002-1016. doi: 10.1111/cid.12824. Epub 2019 Aug 19. Clin Implant Dent Relat Res. 2019. PMID: 31424173 Free PMC article. Clinical Trial.
-
Alloreactivity as therapeutic principle in the treatment of hematologic malignancies. Studies of clinical and immunologic aspects of allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning.Dan Med Bull. 2007 May;54(2):112-39. Dan Med Bull. 2007. PMID: 17521527 Review.
-
Hematopoietic stem cell graft manipulation as a mechanism of immunotherapy.Int Immunopharmacol. 2003 Aug;3(8):1121-43. doi: 10.1016/S1567-5769(03)00014-6. Int Immunopharmacol. 2003. PMID: 12860168 Review.
Cited by
-
Hierarchical Biomaterial Scaffolds for Periodontal Tissue Engineering: Recent Progress and Current Challenges.Int J Mol Sci. 2024 Aug 6;25(16):8562. doi: 10.3390/ijms25168562. Int J Mol Sci. 2024. PMID: 39201249 Free PMC article. Review.
-
Involvement of Root Canal Treatment in Pro-Inflammatory Processes - A Real-World Study.Pragmat Obs Res. 2024 Sep 24;15:165-172. doi: 10.2147/POR.S479124. eCollection 2024. Pragmat Obs Res. 2024. PMID: 39346572 Free PMC article.
References
-
- Lang NP, Berglundh T, Heitz-Mayfield LJ, Pjetursson BE, Salvi GE, Sanz M. Consensus statements and recommended clinical procedures regarding implant survival and complications. Int J Oral Maxillofac Implants. 2004;19(Suppl):150–154. - PubMed
-
- Seibert JS. Reconstruction of deformed, partially edentulous ridges, using full thickness onlay grafts. Part I. technique and wound healing. Compend Contin Educ Dent. 1983;4(6):549–562. - PubMed
-
- Seibert JS. Reconstruction of deformed, partially edentulous ridges, using full thickness onlay grafts. Part II. Prosthetic/periodontal interrelationships. Compend Contin Educ Dent. 1983;4(6):549–562. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

