Highly efficient fermentation of 5-keto-D-fructose with Gluconobacter oxydans at different scales
- PMID: 36496372
- PMCID: PMC9741787
- DOI: 10.1186/s12934-022-01980-5
Highly efficient fermentation of 5-keto-D-fructose with Gluconobacter oxydans at different scales
Abstract
Background: The global market for sweeteners is increasing, and the food industry is constantly looking for new low-caloric sweeteners. The natural sweetener 5-keto-D-fructose is one such candidate. 5-Keto-D-fructose has a similar sweet taste quality as fructose. Developing a highly efficient 5-keto-D-fructose production process is key to being competitive with established sweeteners. Hence, the 5-keto-D-fructose production process was optimised regarding titre, yield, and productivity.
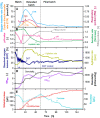
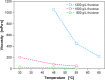
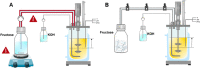
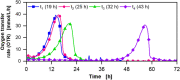
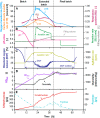
Results: For production of 5-keto-D-fructose with G. oxydans 621H ΔhsdR pBBR1-p264-fdhSCL-ST an extended-batch fermentation was conducted. During fructose feeding, a decreasing respiratory activity occurred, despite sufficient carbon supply. Oxygen and second substrate limitation could be excluded as reasons for the decreasing respiration. It was demonstrated that a short period of oxygen limitation has no significant influence on 5-keto-D-fructose production, showing the robustness of this process. Increasing the medium concentration increased initial biomass formation. Applying a fructose feeding solution with a concentration of approx. 1200 g/L, a titre of 545 g/L 5-keto-D-fructose was reached. The yield was with 0.98 g5-keto-d-fructose/gfructose close to the theoretical maximum. A 1200 g/L fructose solution has a viscosity of 450 mPa∙s at a temperature of 55 °C. Hence, the solution itself and the whole peripheral feeding system need to be heated, to apply such a highly concentrated feeding solution. Thermal treatment of highly concentrated fructose solutions led to the formation of 5-hydroxymethylfurfural, which inhibited the 5-keto-D-fructose production. Therefore, fructose solutions were only heated to about 100 °C for approx. 10 min. An alternative feeding strategy was investigated using solid fructose cubes, reaching the highest productivities above 10 g5-keto-d-fructose/L/h during feeding. Moreover, the scale-up of the 5-keto-D-fructose production to a 150 L pressurised fermenter was successfully demonstrated using liquid fructose solutions (745 g/L).
Conclusion: We optimised the 5-keto-D-fructose production process and successfully increased titre, yield and productivity. By using solid fructose, we presented a second feeding strategy, which can be of great interest for further scale-up experiments. A first scale-up of this process was performed, showing the possibility for an industrial production of 5-keto-D-fructose.
Keywords: 5-Ketofructose; Extended batch fermentation; Fructose dehydrogenase; Gluconobacter oxydans; Scale-up.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Production of the potential sweetener 5-ketofructose from fructose in fed-batch cultivation with Gluconobacter oxydans.Bioresour Technol. 2018 Jul;259:164-172. doi: 10.1016/j.biortech.2018.03.038. Epub 2018 Mar 9. Bioresour Technol. 2018. PMID: 29550669
-
Novel plasmid-free Gluconobacter oxydans strains for production of the natural sweetener 5-ketofructose.Microb Cell Fact. 2020 Mar 4;19(1):54. doi: 10.1186/s12934-020-01310-7. Microb Cell Fact. 2020. PMID: 32131833 Free PMC article.
-
Overexpression of membrane-bound gluconate-2-dehydrogenase to enhance the production of 2-keto-D-gluconic acid by Gluconobacter oxydans.Microb Cell Fact. 2016 Jul 9;15(1):121. doi: 10.1186/s12934-016-0521-8. Microb Cell Fact. 2016. PMID: 27392695 Free PMC article.
-
New developments in oxidative fermentation.Appl Microbiol Biotechnol. 2003 Feb;60(6):643-53. doi: 10.1007/s00253-002-1155-9. Epub 2002 Dec 18. Appl Microbiol Biotechnol. 2003. PMID: 12664142 Review.
-
Glucose oxidation and PQQ-dependent dehydrogenases in Gluconobacter oxydans.J Mol Microbiol Biotechnol. 2009;16(1-2):6-13. doi: 10.1159/000142890. Epub 2008 Oct 29. J Mol Microbiol Biotechnol. 2009. PMID: 18957858 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

