Direct encapsulation of biomolecules in semi-permeable microcapsules produced with double-emulsions
- PMID: 36496516
- PMCID: PMC9736714
- DOI: 10.1038/s41598-022-25895-8
Direct encapsulation of biomolecules in semi-permeable microcapsules produced with double-emulsions
Abstract
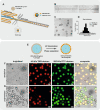
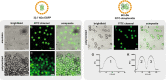
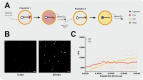
Compartmentalization can serve different purposes such as the protection of biological active substances from the environment, or the creation of a unique combination of biomolecules for diagnostic, therapeutic, or other bioengineering applications. We present a method for direct encapsulation of molecules in biocompatible and semi-permeable microcapsules made from low-molecular weight poly(ethylene glycol) diacrylate (PEG-DA 258). Microcapsules are produced using a non-planar PDMS microfluidic chip allowing for one-step production of water-in-PEG-DA 258-in-water double-emulsions, which are polymerized with UV light into a poly-PEG-DA 258 shell. Semi-permeable microcapsules are obtained by adding an inert solvent to the PEG-DA 258. Due to the favorable hydrophilicity of poly-PEG-DA 258, proteins do not adsorb to the capsule shell, and we demonstrate the direct encapsulation of enzymes, which can also be dried in the capsules to preserve activity. Finally, we leverage capsule permeability for the implementation of a two-layer communication cascade using compartmentalized DNA strand displacement reactions. This work presents the direct encapsulation of active biomolecules in semi-permeable microcapsules, and we expect our platform to facilitate the development of artificial cells and generating encapsulated diagnostics or therapeutics.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Water and Oil Insoluble PEGDA-Based Microcapsule: Biocompatible and Multicomponent Encapsulation.ACS Appl Mater Interfaces. 2018 Nov 28;10(47):40366-40371. doi: 10.1021/acsami.8b16876. Epub 2018 Nov 15. ACS Appl Mater Interfaces. 2018. PMID: 30422614
-
Microfluidic fabrication of polyethylene glycol microgel capsules with tailored properties for the delivery of biomolecules.Biomater Sci. 2017 Jul 25;5(8):1549-1557. doi: 10.1039/c7bm00322f. Biomater Sci. 2017. PMID: 28604857
-
Giant biocompatible and biodegradable PEG-PMCL vesicles and microcapsules by solvent evaporation from double emulsion droplets.J Colloid Interface Sci. 2010 Nov 1;351(1):140-50. doi: 10.1016/j.jcis.2010.05.020. Epub 2010 May 12. J Colloid Interface Sci. 2010. PMID: 20627256
-
Preparation and characterization of biodegradable poly(l-lactide)/poly(ethylene glycol) microcapsules containing erythromycin by emulsion solvent evaporation technique.J Colloid Interface Sci. 2004 Mar 15;271(2):336-41. doi: 10.1016/j.jcis.2003.08.067. J Colloid Interface Sci. 2004. PMID: 14972610
-
Core-shell micro/nanocapsules: from encapsulation to applications.J Microencapsul. 2023 May;40(3):125-156. doi: 10.1080/02652048.2023.2178538. Epub 2023 Feb 28. J Microencapsul. 2023. PMID: 36749629 Review.
Cited by
-
Polydopamine-coated double emulsion capsules for on-demand drug release with reduced passive leakage.Sci Rep. 2025 Aug 24;15(1):31112. doi: 10.1038/s41598-025-15994-7. Sci Rep. 2025. PMID: 40851011 Free PMC article.
-
Functional Scaffolds for Bone Tissue Regeneration: A Comprehensive Review of Materials, Methods, and Future Directions.J Funct Biomater. 2024 Sep 25;15(10):280. doi: 10.3390/jfb15100280. J Funct Biomater. 2024. PMID: 39452579 Free PMC article. Review.
-
Facile encapsulation of cyanoacrylate-based bioadhesive by electrospray method and investigation of the process parameters.Sci Rep. 2024 Mar 5;14(1):5389. doi: 10.1038/s41598-024-56008-2. Sci Rep. 2024. PMID: 38443417 Free PMC article.
References
-
- Datta SS, Abbaspourrad A, Amstad E, Fan J, Kim S-H, Romanowsky M, Shum HC, Sun B, Utada AS, Windbergs M, Zhou S, Weitz DA. 25th anniversary article: Double emulsion templated solid microcapsules: Mechanics and controlled release. Adv. Mater. 2014;26:2205–2218. doi: 10.1002/adma.201305119. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

