Mechanisms of Mitochondrial Apoptosis-Mediated Meat Tenderization Based on Quantitative Phosphoproteomic Analysis
- PMID: 36496558
- PMCID: PMC9741025
- DOI: 10.3390/foods11233751
Mechanisms of Mitochondrial Apoptosis-Mediated Meat Tenderization Based on Quantitative Phosphoproteomic Analysis
Abstract
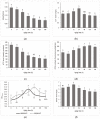
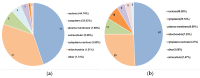
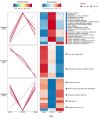
This study investigates the mechanism of phosphorylation in the regulation of apoptosis-mediated meat tenderization during postmortem aging. The results found that the pork muscle exhibited apoptotic potential at early postmortem (48 h) and showed more tenderness at late postmortem, as evidenced by the increase in mitochondrial membrane permeability (MMP), Ca2+ level, reactive oxygen species (ROS) content, and caspases activity at 0 h to 48 h, and decreases in ATP level at 0 h to 24 h and shear force at 12 h to 120 h (p < 0.05). Phosphoproteomic analysis revealed that phosphorylation regulated apoptosis by modulating ATP and calcium bindings as well as apoptotic signaling, which occurred within early 12 h and mainly occurred at 12 h to 48 h postmortem. Moreover, differential expression of phosphoproteins demonstrated that phosphorylation regulated oxidative stress-induced apoptosis and rigor mortis, thereby promoting the development of meat tenderness. Our results provide insights into the roles of phosphorylation in various physiological processes that affect meat tenderness.
Keywords: apoptosis; muscle; postmortem; proteins; quantitative phosphoproteomic; tenderness.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Effect of oxidative stress on AIF-mediated apoptosis and bovine muscle tenderness during postmortem aging.J Food Sci. 2020 Jan;85(1):77-85. doi: 10.1111/1750-3841.14969. Epub 2019 Dec 9. J Food Sci. 2020. PMID: 31816098
-
Quantitative phosphoproteomic analysis of porcine muscle within 24 h postmortem.J Proteomics. 2014 Jun 25;106:125-39. doi: 10.1016/j.jprot.2014.04.020. Epub 2014 Apr 24. J Proteomics. 2014. PMID: 24769528
-
The correlation between Smac, IAPs and mitochondrial apoptosis, muscle tenderness during postmortem aging of Oula Tibetan sheep meat.Food Chem X. 2024 Oct 9;24:101887. doi: 10.1016/j.fochx.2024.101887. eCollection 2024 Dec 30. Food Chem X. 2024. PMID: 39498258 Free PMC article.
-
Apoptosis and its role in postmortem meat tenderness: A comprehensive review.Meat Sci. 2025 Jan;219:109652. doi: 10.1016/j.meatsci.2024.109652. Epub 2024 Sep 8. Meat Sci. 2025. PMID: 39265386 Review.
-
Contribution of mitochondria to postmortem muscle tenderization: a review.Crit Rev Food Sci Nutr. 2025;65(1):30-46. doi: 10.1080/10408398.2023.2266767. Epub 2023 Oct 11. Crit Rev Food Sci Nutr. 2025. PMID: 37819615 Review.
Cited by
-
Apoptosis and autophagy of muscle cell during pork postmortem aging.Anim Biosci. 2024 Feb;37(2):284-294. doi: 10.5713/ab.23.0148. Epub 2023 Oct 27. Anim Biosci. 2024. PMID: 37905320 Free PMC article.
References
-
- Ouali A., Gagaoua M., Boudida Y., Becila S., Boudjellal A., Herrera-Mendez C.H., Sentandreu M.A. Biomarkers of meat tenderness: Present knowledge and perspectives in regards to our current understanding of the mechanisms involved. Meat Sci. 2013;95:854–870. doi: 10.1016/j.meatsci.2013.05.010. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

