Natural Mycoplasma Infection Reduces Expression of Pro-Inflammatory Cytokines in Response to Ovine Footrot Pathogens
- PMID: 36496756
- PMCID: PMC9737833
- DOI: 10.3390/ani12233235
Natural Mycoplasma Infection Reduces Expression of Pro-Inflammatory Cytokines in Response to Ovine Footrot Pathogens
Abstract
Ovine footrot is a complex multifactorial infectious disease, causing lameness in sheep with major welfare and economic consequences. Dichelobacter nodosus is the main causative bacterium; however, footrot is a polymicrobial disease with Fusobacterium necrophorum, Mycoplasma fermentans and Porphyromonas asaccharolytica also associated. There is limited understanding of the host response involved. The proinflammatory mediators, interleukin (IL)-1β and C-X-C Motif Chemokine Ligand 8 (CXCL8), have been shown to play a role in the early response to D. nodosus in dermal fibroblasts and interdigital skin explant models. To further understand the response of ovine skin to bacterial stimulation, and to build an understanding of the role of the cytokines and chemokines identified, primary ovine interdigital fibroblasts and keratinocytes were isolated, cultured and stimulated. The expression of mRNA and protein release of CXCL8 and IL-1β were measured after stimulation with LPS, D. nodosus or F. necrophorum, which resulted in increased transcript levels of IL-1β and CXCL8 in the M. fermentans-free cells. However, only an increase in the CXCL8 protein release was observed. No IL-1β protein release was detected, despite increases in IL-1β mRNA, suggesting the signal for intracellular pre-IL-1β processing may be lacking when culturing primary cells in isolation. The keratinocytes and fibroblasts naturally infected with M. fermentans showed little response to the LPS, a range of D. nodosus preparations or heat-inactivated F. necrophorum. Primary single cell culture models complement ex vivo organ culture models to study different aspects of the host response to D. nodosus. The ovine keratinocytes and fibroblasts infected with M. fermentans had a reduced response to the experimental bacterial stimulation. However, in the case of footrot where Mycoplasma spp. are associated with diseased feet, this natural infection gives important insights into the impact of multiple pathogens on the host response.
Keywords: cell culture; footrot; sheep.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


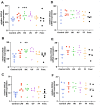
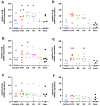

Similar articles
-
A Novel 3D Skin Explant Model to Study Anaerobic Bacterial Infection.Front Cell Infect Microbiol. 2017 Sep 14;7:404. doi: 10.3389/fcimb.2017.00404. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28959685 Free PMC article.
-
Characterisation of Dichelobacter nodosus and detection of Fusobacterium necrophorum and Treponema spp. in sheep with different clinical manifestations of footrot.Vet Microbiol. 2015 Aug 31;179(1-2):82-90. doi: 10.1016/j.vetmic.2015.02.034. Epub 2015 Mar 7. Vet Microbiol. 2015. PMID: 25796133
-
Immunological response of lame sheep to clinical interdigital dermatitis and footrot: Procalcitonin, acute phase proteins, and pro-inflammatory cytokines.Comp Immunol Microbiol Infect Dis. 2022 Nov-Dec;90-91:101899. doi: 10.1016/j.cimid.2022.101899. Epub 2022 Oct 27. Comp Immunol Microbiol Infect Dis. 2022. PMID: 36343421
-
Aetiology, Risk Factors, Diagnosis and Control of Foot-Related Lameness in Dairy Sheep.Animals (Basel). 2019 Jul 31;9(8):509. doi: 10.3390/ani9080509. Animals (Basel). 2019. PMID: 31370310 Free PMC article. Review.
-
Ovine footrot: A review of current knowledge.Vet J. 2021 May;271:105647. doi: 10.1016/j.tvjl.2021.105647. Epub 2021 Feb 22. Vet J. 2021. PMID: 33840488 Review.
Cited by
-
3D Approaches to Culturing Bovine Skin: Explant Culture versus Organotypic Skin Model.Cells Tissues Organs. 2024;213(5):424-438. doi: 10.1159/000538438. Epub 2024 Mar 21. Cells Tissues Organs. 2024. PMID: 38508156 Free PMC article.
References
-
- Prosser N.S., Purdy K.J., Green L.E. Increase in the flock prevalence of lameness in ewes is associated with a reduction in farmers using evidence-based management of prompt treatment: A longitudinal observational study of 154 English sheep flocks 2013–2015. Prev. Vet. Med. 2019;173:104801. doi: 10.1016/j.prevetmed.2019.104801. - DOI - PMC - PubMed
-
- Kennan R.M., Gilhuus M., Frosth S., Seemann T., Dhungyel O.P., Whittington R.J., Boyce J.D., Powell D.R., Aspán A., Jørgensen H.J., et al. Genomic Evidence for a Globally Distributed, Bimodal Population in the Ovine Footrot Pathogen Dichelobacter nodosus. mBio. 2014;5:e01821-14. doi: 10.1128/mBio.01821-14. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

